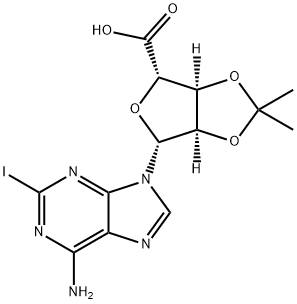
5'-Carboxy-2-iodo-2',3'-O-isopropylidene-D-adenosine synthesis
- Product Name:5'-Carboxy-2-iodo-2',3'-O-isopropylidene-D-adenosine
- CAS Number:141018-26-0
- Molecular formula:C13H14IN5O5
- Molecular Weight:447.19
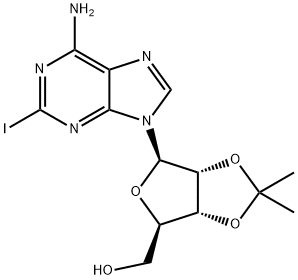
141018-25-9
21 suppliers
$110.00/10mg

141018-26-0
19 suppliers
$85.00/5mg
Yield:141018-26-0 95%
Reaction Conditions:
with 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;[bis(acetoxy)iodo]benzene in water;acetonitrile at 0 - 20; for 22.5 h;Jones Oxidation;
Steps:
A.A2; 2 SCHEME A; step A2; Example 2: Synthesis of 1'-deoxy-1'-(6-amino-2-iodo-9H-purin-9-yl)-2',3'-O-isopropylidene-β-D-ribofuranuronic acid (Compound 3)
To a solution of Compound 2 (10.0 g, 23.1 mmol) in CH3CN (200 mL) and water (50 mL) cooled to 0°C was added iodobenzene diacetate (16.4 g, 50.8 mmol) and TEMPO (0.72 g, 20 mmol). The mixture was stirred at 0°C for 30 minutes, then allowed to warm to room temperature and stirred for 22 h. The solvents were evaporated and the resulting residue was triturated with n-heptane (400 mL) overnight. The solids were filtered, washed with n-heptane and dried III vacuo to afford Compound 3 (9.80 g, 95%) as an off-white solid. H-NMR (600 MHz, CD30D): 1.45 (s, 3H), 1.65 (S, 3H), 4. 78 (d, 1H), 5.50 (d, 1H), 5.67 (dd, 1H), 6.31 (s, 1H), 8.14 (s, 1H) ; 13C-NMR (150 MHz, CD30D) : 25.45, 27.08, 85. 69, 86.09, 88.44, 92.59, 114.92, 120.39, 142.47, 151.08, 157. 20,173. 14. LRMS (ES): 448.0 (M+H, 100%).
References:
WO2004/104017,2004,A2 Location in patent:Page 18; 32
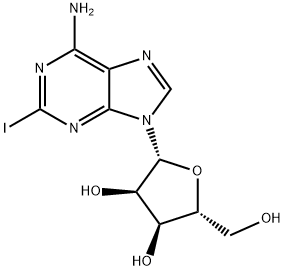
35109-88-7
193 suppliers
$41.00/100mg

141018-26-0
19 suppliers
$85.00/5mg
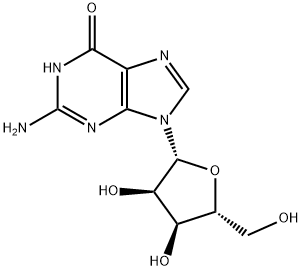
118-00-3
657 suppliers
$5.00/10g

141018-26-0
19 suppliers
$85.00/5mg
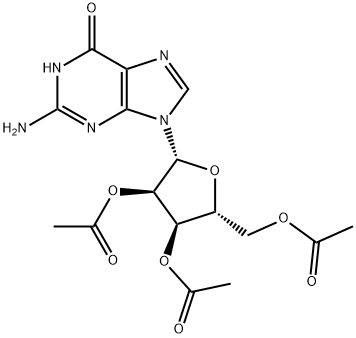
6979-94-8
230 suppliers
$10.00/1g

141018-26-0
19 suppliers
$85.00/5mg
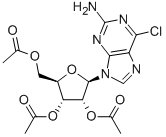
16321-99-6
98 suppliers
$20.00/250mg

141018-26-0
19 suppliers
$85.00/5mg