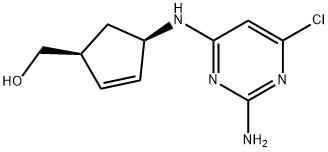
(1S,4R)-4-[(2,5-DiaMino-6-chloro-4-pyriMidinyl)aMino]-2-cyclopentene-1-Methanol synthesis
- Product Name:(1S,4R)-4-[(2,5-DiaMino-6-chloro-4-pyriMidinyl)aMino]-2-cyclopentene-1-Methanol
- CAS Number:141271-11-6
- Molecular formula:C10H13ClN4O
- Molecular Weight:240.69
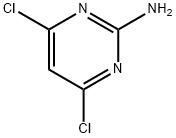
56-05-3
447 suppliers
$10.00/1g
![(1S,4R)-2-Aza-bicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/130931-83-8.gif)
130931-83-8
171 suppliers
$8.00/100mg
![(1S,4R)-4-[(2,5-DiaMino-6-chloro-4-pyriMidinyl)aMino]-2-cyclopentene-1-Methanol](/CAS/GIF/141271-11-6.gif)
141271-11-6
13 suppliers
$165.00/2.5mg
Yield:141271-11-6 75%
Reaction Conditions:
Stage #1: (1S,4R)-2-azabicyclo[2,2,1]hept-5-en-3-onewith methanesulfonic acid in tetrahydrofuran;water at 62 - 65; for 3 h;
Stage #2: with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 22; for 22.58 h;Heating / reflux;
Stage #3: 2-Amino-4,6-dichloropyrimidinewith sodium hydroxide;sodium fluoride;triethylaminemore than 3 stages;
Steps:
29 EXAMPLE 29. (1S, 4R)-[2-(2-Amino-6-chloro-4-pyrimidinyl)amino]-2-cyclopentene-1-methanol
A solution of (-)-2-azabicyclo[2.2.1hept-5-en-3-one (Enzymatix lot No.LN 1253, 30.0 g, 275 mmol) in anhydrous tetrahydrofuran (150 mL) in a 2 L 3-neck round bottom flask under nitrogen was equipped with a thermometer and mechanical stirrer, then warmed to 35° C. (most of solid dissolved). Meanwhile, a solution of methanesulfonic acid (28.0 g, 291 mmol) and water (5.35 g, 297 mmol) in tetrahydrofuran (50 mL) was prepared (caution-mixing is highly exothermic). This solution was slowly added dropwise via an addition funnel to the 2 L flask over 10 min. Initially the solution became turbid, and bythe end of the addition some solid had appeared on the side of the flask. The mixture was heated to gentle reflux for 3 h (internal temperature 62-65° C.), then cooled (-15° C.). An air-dried sample of the white solid had 1H-NMR identical with the sample of (-)-(1S, 4R)-4-amino-2-cyclopentene-1-carboxylic acid methanesulfonate described in Example 1. A solution of 1.0 N lithium aluminurm hydride in tetrahydrofuran (Aldrich, 525 mL, 525 mmol) was added dropwise to the mixture (slowly at first, more rapidly later) so that pot temperature remained below 0° C. After addition was complete (required approx. 35 min) the mixture was slowly warmed to 22° C. and stirred at room temperature for 17 h, then refluxed for 5 h and cooled to ambient. Sodium fluoride (150 g, 3.57 mol) was added, stirring was continued for 30 min, then the mixture was cooled on an ice bath (5° C.). Water (38 g, 2.1 mol) was added dropwise so that pot temperature remained below 20° C. (over 30 min), then the mixture was stirred at room temperature for 20 m inand filtered. The filter cake was washed with tetrahydrofuranimethanol (5:2), and the filtrate was set aside. The filter cake was taken up in tetrahydrofuranimethanol (5:2, 700 mL), stirred for 15 min, and filtered. This extraction was repeated and the three filtrates were combined and cooled (0° C.), then refiltered. A 1 mL aliquot of this solution was concentrated to give (-)-(1S, 4R)-4-amino-2-cyclopentene-1-methanol as a colorless oil with identical analysis to the sample described in Example 2 and 3. The remainder of the solution was partially concentrated in vacuo, diluted with 1-butanol (500 mL), further concentrated to remove tetrahydrofuran and methanol, and transferred to a 1 L 3-neck flask under nitrogen equipped with a thermometer and reflux condenser. Triethylamine (125 mL. 900 mmol) and 2-amino-4,6-dichloropynmidine (47.0 g, 286 mmol) were added, and the mixture was refluxed for 4 h (internal temperature 107-108° C.). The reaction solution was partially concentrated in vacuo and treated with 5N sodium hydroxide (60 mL, 300 mmol). The solution was concentrated in vacua, diluted with toluene (100 mL), and further concentrated in order to remove the remaining triethylamine. The residual oil was taken up in chloroform (500 mL) and methanol (100 mL), then the mixture was filtered. The filter cake was washed with methanol/chloro-form (1:9), then the filtrate was concentrated in vacuo and the residual oil dissoived in chloroform and loaded onto a column of silica gel containing 300 g of silica. The column was initially eluted with 3% ethanol/chloroform, then with 8% ethanol/chloroform to afford pure fractions of the subject compound; these were concentrated in vacuo to constant weight to afford (1S, 4R)-[2-(2-amino-6-chloro-4-pyrimidinyl)amino]-2cyclopentene-1-methanol as a pale tan gum (53.1 g, 75%); mp 73-75° C. as a pale tan solid hydrate (methanollwater). 1H-NMR (DMSO-d6): 7.00-7.10 (br s, 1H); 6.35-6.45(br s, 2H); 5.87 (m, 1H); 5.73 (s, 1H); 5.71 (m, 1H); 4.90-4.05 (br s, 1H); 4.64 (t, 1H, J=5Hz); 3.36 (m, 2H); 2.6-2.75 (m, 1H); 2.30-2.40 (m, 1H); 1.20-1.30 (m, 1H). Ms (Cl): m/z 241 (m+H+, 100). [α]20589-27.3° (c=0.54, methanol. Anal. Calcd for C10H13ClN4O·H2O: C, 46.43; H, 5.84; N, 21.66. Found: C, 46.49; H, 5.81; N. 21.79.
References:
US6392085,2002,B1 Location in patent:Page column 31-32

56-05-3
447 suppliers
$10.00/1g

71-36-3
1091 suppliers
$14.00/25mL
![(1S,4R)-4-[(2,5-DiaMino-6-chloro-4-pyriMidinyl)aMino]-2-cyclopentene-1-Methanol](/CAS/GIF/141271-11-6.gif)
141271-11-6
13 suppliers
$165.00/2.5mg

56-05-3
447 suppliers
$10.00/1g
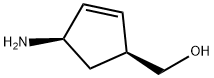
136522-35-5
87 suppliers
$51.03/Price $/50mgs:
![(1S,4R)-4-[(2,5-DiaMino-6-chloro-4-pyriMidinyl)aMino]-2-cyclopentene-1-Methanol](/CAS/GIF/141271-11-6.gif)
141271-11-6
13 suppliers
$165.00/2.5mg
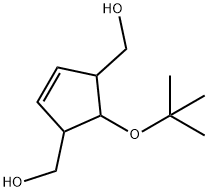
182689-49-2
0 suppliers
inquiry
![(1S,4R)-4-[(2,5-DiaMino-6-chloro-4-pyriMidinyl)aMino]-2-cyclopentene-1-Methanol](/CAS/GIF/141271-11-6.gif)
141271-11-6
13 suppliers
$165.00/2.5mg
![Carbamic acid, [4-(hydroxymethyl)-2-cyclopenten-1-yl]-, methyl ester, (1R-cis)- (9CI)](/CAS/20210305/GIF/182689-66-3.gif)
182689-66-3
0 suppliers
inquiry
![(1S,4R)-4-[(2,5-DiaMino-6-chloro-4-pyriMidinyl)aMino]-2-cyclopentene-1-Methanol](/CAS/GIF/141271-11-6.gif)
141271-11-6
13 suppliers
$165.00/2.5mg