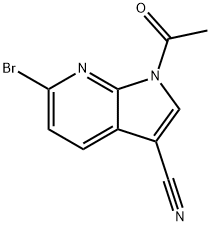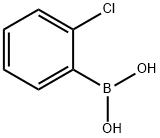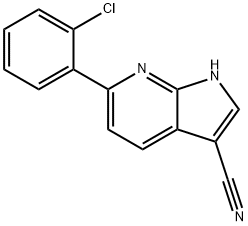
6-(2-Chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonitrile synthesis
- Product Name:6-(2-Chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonitrile
- CAS Number:1413066-31-5
- Molecular formula:C14H8ClN3
- Molecular Weight:253.69
Yield:1413066-31-5 59%
Reaction Conditions:
with triethylamine;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;XPhos in 1,4-dioxane at 100;Inert atmosphere;
Steps:
47.1
Step 1. In a 100 mL round-bottomed flask, 1-acetyl-6-bromo-1H-pyrrolo[2,3-b]pyridine-3-carbonitrile (250 mg, 947 μmol, Eq: 1.00), triethylamine (575 mg, 792 μl, 5.68 mmol, Eq: 6) and X-PHOS (181 mg, 379 μmol, Eq: 0.40) were combined with dioxane (25.0 ml) to give a colorless solution. [1,1'-bis(diphenylphosphino)ferrocene]dichloropaladium(II) (173 mg, 237 μmol, Eq: 0.25) and 2-chlorophenylboronic acid (296 mg, 1.89 mmol) were added and the resultant mixture was degassed for 5 minutes under Nitrogen. The reaction mixture was heated to 100° C. and stirred overnight. The reaction mixture was poured into 50 mL H2O and extracted with EtOAc (3×50 mL). The organic layers were dried over MgSO4 and concentrated in vacuo. The crude material was purified by flash chromatography (silica gel, 40 g, 20% to 30% EtOAc in hexanes) to give 6-(2-chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonitrile (142 mg, 59%).
References:
US2012/295885,2012,A1 Location in patent:Page/Page column 66