
(S)-3-[5-(3,5-dichloro-4-fluoro-phenyl)-5-trifluoromethyl-4,5-dihydro-isoxazol-3-yl]-5,6-dihydro-4H-cyclopenta[c]thiophene-1-carboxylic acid [(2,2-difluoro-ethylcarbamoyl)-methyl]-amide synthesis
- Product Name:(S)-3-[5-(3,5-dichloro-4-fluoro-phenyl)-5-trifluoromethyl-4,5-dihydro-isoxazol-3-yl]-5,6-dihydro-4H-cyclopenta[c]thiophene-1-carboxylic acid [(2,2-difluoro-ethylcarbamoyl)-methyl]-amide
- CAS Number:1414642-93-5
- Molecular formula:C22H17Cl2F6N3O3S
- Molecular Weight:588.35
![4H-Cyclopenta[c]thiophene-1-carboxamide, 3-[5-(3,5-dichloro-4-fluorophenyl)-4,5-dihydro-5-(trifluoromethyl)-3-isoxazolyl]-N-[2-[(2,2-difluoroethyl)amino]-2-oxoethyl]-5,6-dihydro-](/CAS/20210305/GIF/1414642-56-0.gif)
1414642-56-0
0 suppliers
inquiry
![(S)-3-[5-(3,5-dichloro-4-fluoro-phenyl)-5-trifluoromethyl-4,5-dihydro-isoxazol-3-yl]-5,6-dihydro-4H-cyclopenta[c]thiophene-1-carboxylic acid [(2,2-difluoro-ethylcarbamoyl)-methyl]-amide](/CAS/20180703/GIF/1414642-93-5.gif)
1414642-93-5
3 suppliers
inquiry
Yield:1414642-93-5 93%
Reaction Conditions:
with Chiralcel AD-H column in ethanol;carbon dioxide;Purification / work up;Resolution of racemate;
Steps:
245
Example 245(5)-3-[5-(3,5-dichloro-4-fluoro-phenyl)-5-trifluoromethyl-4,5-dihydro- isoxazol-3-yl]-5,6-dihydro-4H-cyclopenta[c]thiophene-l-carboxylic acid[(2,2-difluoro-ethylcarbamoyl)-methyl]-amide3 g of 3-[5-(3,5-dichloro-4-fluoro-phenyl)-5-trifluoromethyl-4,5-dihydro- isoxazol-3-yl]-5,6-dihydro-4H-cyclopenta[c]thiophene-l-carboxylic acid[(2,2-difluoro-ethylcarbamoyl)-methyl]-amide is separated by SFC separation to give desired product (1.4 g, 93 %).1H NMR (CDC13, 400 MHz): δ 7.56 (d, J=6.0, 2 H), 6.64 (brs, 1 H), 6.40 (brs, 1 H), 6.03-5.73 (m, 1 H), 4.15 (d, J=5.2, 2 H), 4.01 (d, J=17.2, 1 H), 3.74-3.65 (m, 1 H), 3.62 (d, J=17.2, 1 H), 2.97 (t, J=7.6, 2 H), 2. 89 (t, J=7.6, 2 H), 2.56 (m, 2 H). SFC conditions are as follows:Instrument: Thar 350Column: AD 250mm*50mm, lOumMobile phase: A: Supercritical C02, B: EtOH, A:B =60:40 at 240ml/minColumn Temp: 38 °CNozzle Pressure: lOOBarNozzle Temp: 60 °CEvaporator Temp: 20 °CTrimmer Temp: 25 °CWavelength: 220nm
References:
WO2012/155676,2012,A1 Location in patent:Page/Page column 78-79

120-92-3
427 suppliers
$11.00/25g
![(S)-3-[5-(3,5-dichloro-4-fluoro-phenyl)-5-trifluoromethyl-4,5-dihydro-isoxazol-3-yl]-5,6-dihydro-4H-cyclopenta[c]thiophene-1-carboxylic acid [(2,2-difluoro-ethylcarbamoyl)-methyl]-amide](/CAS/20180703/GIF/1414642-93-5.gif)
1414642-93-5
3 suppliers
inquiry
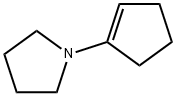
7148-07-4
95 suppliers
$57.79/10g
![(S)-3-[5-(3,5-dichloro-4-fluoro-phenyl)-5-trifluoromethyl-4,5-dihydro-isoxazol-3-yl]-5,6-dihydro-4H-cyclopenta[c]thiophene-1-carboxylic acid [(2,2-difluoro-ethylcarbamoyl)-methyl]-amide](/CAS/20180703/GIF/1414642-93-5.gif)
1414642-93-5
3 suppliers
inquiry
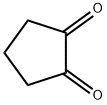
3008-40-0
29 suppliers
$315.00/100mg
![(S)-3-[5-(3,5-dichloro-4-fluoro-phenyl)-5-trifluoromethyl-4,5-dihydro-isoxazol-3-yl]-5,6-dihydro-4H-cyclopenta[c]thiophene-1-carboxylic acid [(2,2-difluoro-ethylcarbamoyl)-methyl]-amide](/CAS/20180703/GIF/1414642-93-5.gif)
1414642-93-5
3 suppliers
inquiry
![Acetic acid, 2-[(2-oxopropyl)thio]-, Methyl ester](/CAS/20150408/GIF/61363-62-0.gif)
61363-62-0
6 suppliers
inquiry
![(S)-3-[5-(3,5-dichloro-4-fluoro-phenyl)-5-trifluoromethyl-4,5-dihydro-isoxazol-3-yl]-5,6-dihydro-4H-cyclopenta[c]thiophene-1-carboxylic acid [(2,2-difluoro-ethylcarbamoyl)-methyl]-amide](/CAS/20180703/GIF/1414642-93-5.gif)
1414642-93-5
3 suppliers
inquiry