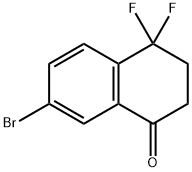
7-BROMO-4,4-DIFLUORO-3,4-DIHYDRONAPHTHALEN-1(2H)-ONE synthesis
- Product Name:7-BROMO-4,4-DIFLUORO-3,4-DIHYDRONAPHTHALEN-1(2H)-ONE
- CAS Number:1415108-26-7
- Molecular formula:C10H7BrF2O
- Molecular Weight:261.06

1415108-25-6
2 suppliers
inquiry

1415108-26-7
19 suppliers
inquiry
Yield:1415108-26-7 71%
Reaction Conditions:
with potassium permanganate;potassium dihydrogenphosphate;trisodium phosphate*7H2O in water;tert-butyl alcohol at 20; for 15 h;
Steps:
c
c) 7-Bromo-4,4-difluoro-3,4-dihydro-2H-naphthalen-1-one A solution of 6-bromo-1,1-difluoro-1,2,3,4-tetrahydro-naphthalene (691 mg, 2.8 mmol) in tert-butanol (7 ml). A solution of potassium dihydrogenphosphate (769 mg, 5.59 mmol) in water (2 ml) and a solution of sodium phosphate heptahydrate (1.51 g, 5.59 mmol) in water (2 ml) were added. Thereafter, potassium permanganate (670 mg, 4.2 mmol) was added and the reaction mixture stirred at room temperature for 15 hours. For the workup, the mixture was diluted with ethyl acetate (200 ml), the organic layer separated, washed with water (10 ml) and brine (10 ml), finally dried over sodium sulphate and evaporated at reduced pressure. The crude product was purified by chromatography on silica gel using a gradient of heptane/ethyl acetate=100:0 to 80:20 as the eluent. The 7-bromo-4,4-difluoro-3,4-dihydro-2H-naphthalen-1-one (515 mg, 71% yield) was obtained as a colorless oil.
References:
US2012/302549,2012,A1 Location in patent:Page/Page column 30
![Spiro[1,3-dithiolane-2,1'(2'H)-naphthalene], 6'-bromo-3',4'-dihydro-](/CAS/20210305/GIF/1415108-24-5.gif)
1415108-24-5
2 suppliers
inquiry

1415108-26-7
19 suppliers
inquiry
66361-67-9
170 suppliers
$50.00/100mg

1415108-26-7
19 suppliers
inquiry