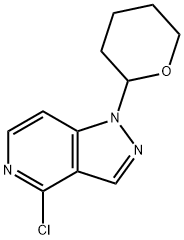
1H-Pyrazolo[4,3-c]pyridine,4-chloro-1-(tetrahydro-2H-pyran-2-yl) synthesis
- Product Name:1H-Pyrazolo[4,3-c]pyridine,4-chloro-1-(tetrahydro-2H-pyran-2-yl)
- CAS Number:1416713-66-0
- Molecular formula:C11H12ClN3O
- Molecular Weight:237.69
110-87-2
543 suppliers
$10.00/1g
871836-51-0
168 suppliers
$30.00/1g
![1H-Pyrazolo[4,3-c]pyridine,4-chloro-1-(tetrahydro-2H-pyran-2-yl)](/CAS/20180703/GIF/1416713-66-0.gif)
1416713-66-0
8 suppliers
inquiry
Yield:1416713-66-0 89%
Reaction Conditions:
with toluene-4-sulfonic acid in dichloromethane at 20; for 24 h;
Steps:
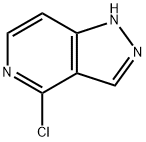
P3 4-Chloro-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazolo[4,3-c]pyridine (P3)
Preparation P3
4-Chloro-1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazolo[4,3-c]pyridine (P3)
p-Toluenesulfonic acid monohydrate (2.4 g, 13 mmol) and 3,4-dihydro-2H-pyran (99%, 45 mL, 520 mmol) were sequentially added to a suspension of 4-chloro-1H-pyrazolo[4,3-c]pyridine (20.0 g, 130 mmol) in dichloromethane (400 mL).
The reaction mixture was allowed to stir at room temperature for 24 hours, at which time it was washed with saturated aqueous sodium bicarbonate solution.
The organic layer was dried over sodium sulfate, filtered, and concentrated in vacuo.
Purification via silica gel chromatography (Eluents: 10%, then 30%, then 50% ethyl acetate in heptane) afforded the product as a white solid. Yield: 27.51 g, 115.7 mmol, 89%. LCMS m/z 238.1 [M+H+].
1H NMR (400 MHz, CDCl3) δ 8.19 (d, J=6.0 Hz, 1H), 8.16 (d, J=0.9 Hz, 1H), 7.47 (dd, J=6.0, 0.9 Hz, 1H), 5.73 (br dd, J=9.0, 2.7 Hz, 1H), 3.97-4.04 (m, 1H), 3.72-3.80 (m, 1H), 2.43-2.53 (m, 1H), 2.07-2.20 (m, 2H), 1.65-1.85 (m, 3H).
References:
US2014/128374,2014,A1 Location in patent:Paragraph 0317; 0318