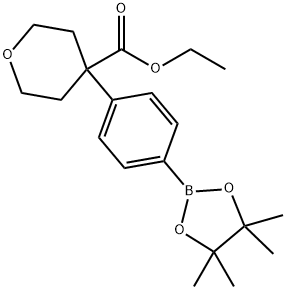
ethyl 4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)tetrahydro-2H-pyran-4-carboxylate synthesis
- Product Name:ethyl 4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)tetrahydro-2H-pyran-4-carboxylate
- CAS Number:1423702-59-3
- Molecular formula:C20H29BO5
- Molecular Weight:360.25

1227160-22-6
7 suppliers
inquiry

73183-34-3
587 suppliers
$6.00/5g

1423702-59-3
13 suppliers
inquiry
Yield:1423702-59-3 68%
Reaction Conditions:
with potassium acetate in 1,4-dioxane at 110;Inert atmosphere;
Steps:
2 Step 2: Ethyl 4-(4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)phenyl)tetrah dro-2//-pyran- 4-carboxylate
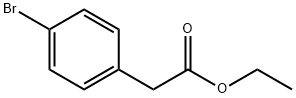
A mixture of ethyl 4-(4-bromophenyl)tetrahydropyran-4-carboxylate (4.5 g, 14.4 mmol), (1374) 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(l,3,2-dioxaborolane) (3.65 g, 14.4 mmol), KOAc (2.82 g, 28.7 mmol) and l,4-dioxane (60 mL) was evacuated-purged with nitrogen three times. Pd(dppf)Cl2 (526 mg, 0.72 mmol) was added and the mixture stirred at 1 l0°C overnight under a nitrogen atmosphere. The reaction mixture was cooled to room temperature, poured into water (80 mL) and then extracted with EtOAc (3 x30 mL). The combined organic layers were dried over Na2S0 , filtered, concentrated, and purified by column chromatography (Si02, petroleum ether/ethyl acetate=30/l) to give ethyl 4-(4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)phenyl)tetrahydro-2i7-pyran-4- carboxylate (3.5 g, 68%) as a white solid. 1H NMR (400 MHz, CDCl3): d 7.80 (d, 2H), 7.38 (d, 2H), 4.13 (q, 2H), 3.93 (d, 2H), 3.59 (t, 2H), 2.61-2.45 (m, 2H), 2.08-1.92 (m, 2H), 1.31 (s, 12H), 1.18 (t, 3H); LCMS: 361.3 [M+H]+.
References:
WO2019/241751,2019,A1 Location in patent:Paragraph 00514
14062-25-0
255 suppliers
$6.00/5g

1423702-59-3
13 suppliers
inquiry