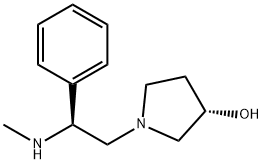
(2'S,3S)-1-(2-METHYLAMINO-2-PHENYL-ETHYL)-PYRROLIDIN-3-OL synthesis
- Product Name:(2'S,3S)-1-(2-METHYLAMINO-2-PHENYL-ETHYL)-PYRROLIDIN-3-OL
- CAS Number:142773-73-7
- Molecular formula:C13H20N2O
- Molecular Weight:220.31
![Carbamic acid, N-?[(1S)?-?2-?[(3S)?-?3-?hydroxy-?1-?pyrrolidinyl]?-?2-?oxo-?1-?phenylethyl]?-?, ethyl ester](/CAS/20210111/GIF/207847-98-1.gif)
207847-98-1
1 suppliers
inquiry

142773-73-7
31 suppliers
$187.00/0.25g
Yield:142773-73-7 90%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0; for 3 h;Reflux;
Steps:
9.iii Step-ui: Synthesis of (S)- I -((S)-2-(methylamino)-2-phenylethyl)pyffolidin-3-ol
LAH (0.78g. 20.5rnmol) was dissolved in dry THF (10m1) at 0-5°C, followed by addition of ((S)-2-((S)-3 -hydroxypyrrolidin- 1 -yl)-2-oxo- 1 -phenylethyl) (1 . 5g,5.l3mmol) in THF (lOmi). The reaction mixture was refluxed for 3h, quenched with saturated Na2CO3 solution and triturated with ethyl acetate. The reaction mixture was filtered through celite and organic layer was concentrated under reduce pressure to get the (S)-1-((S)-2-(methylamino)-2-phenylethyl)pyrroliclin-3-ol, as a pale yellow oil. (1.02, 90% yield).‘H NMR: (DMSO-d6, 400 MHz): 7.37-7.27 (m, 4H), 7.26-7.23 (m, 1H), 4.80-4.68 (m, IH), 4.27-4.16 (m, 1H), 3.69-3.62 (m, 2H), 2.78-2.62 (m, 2H), 2.52 (s, 3H), 2.25-2.12 (m, 2H), 1.77-1.64 (m, 2H); ESI-MS: (+ve mode) 221.05 (M+H) (100 %).
References:
WO2015/114660,2015,A1 Location in patent:Page/Page column 25
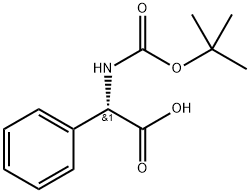
2900-27-8
290 suppliers
$10.00/1g

142773-73-7
31 suppliers
$187.00/0.25g
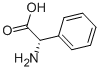
2935-35-5
405 suppliers
$5.00/10g

142773-73-7
31 suppliers
$187.00/0.25g
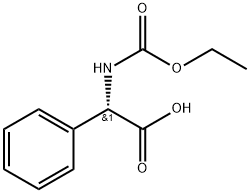
60729-80-8
13 suppliers
inquiry

142773-73-7
31 suppliers
$187.00/0.25g