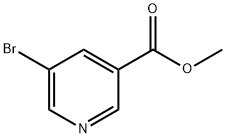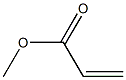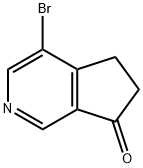
4-BROMO-5H-CYCLOPENTA[C]PYRIDIN-7(6H)-ONE(WXC07919) synthesis
- Product Name:4-BROMO-5H-CYCLOPENTA[C]PYRIDIN-7(6H)-ONE(WXC07919)
- CAS Number:1428651-90-4
- Molecular formula:C8H6BrNO
- Molecular Weight:212.04
Yield:1428651-90-4 817 mg
Reaction Conditions:
Stage #1: 5-bromo-3-pyridine carboxylic acid methyl esterwith n-butyllithium;diisopropylamine in tetrahydrofuran at -78; for 0.5 h;
Stage #2: acrylic acid methyl ester in tetrahydrofuran at -78; for 1.5 h;
Steps:
A
Step A: A solution of diisopropylamine (3.37 mL, 23.9 mmol) in 100 mL of THF is cooled to 0°C and then treated with n-butyllithium (11.95 mL, 23.9 mmol). The mixture is stuffed at 0°C for 15 minutes then cooled to -78°C. Methyl 5-bromonicotinate (4.70 g, 21.7 mmol) is added as solution in 20 mL of THF drop-wise. The mixture is stuffed at -78°C for 30 minutes then treated with methyl acrylate (4.89 mL, 54.3 mmol) in 20 mL of THF drop-wise. The mixture is stirred at -78°C for 1.5 hours then quenched with 50 mL of 10% acetic acid. The reaction mixture is evaporated to dryness. The crude solid is treated with 54 mL of 6N HC1 and stirred at 100°C for 1 hour. The reaction mixture is cooled in ice, basified to pH 7-8 with 5N NaOH and extracted twice with EtOAc. The combined org layer is washed with brine, dried over MgSO4, filtered and concentrated. Purification by silica gel flash column chromatography eluting with 20-50% EtOAc/heptane affords 817 mg of 4-bromo-5,6-dihydro-[2]pyrindin-7-one.
References:
WO2016/61161,2016,A1 Location in patent:Page/Page column 47; 48
1440520-08-0
1 suppliers
inquiry
![4-BROMO-5H-CYCLOPENTA[C]PYRIDIN-7(6H)-ONE(WXC07919)](/CAS/20180906/GIF/1428651-90-4.gif)
1428651-90-4
29 suppliers
inquiry
![5H-Cyclopenta[c]pyridine-6-carboxylic acid, 4-bromo-7-hydroxy-, methyl ester, lithium salt (1:1)](/CAS/20211123/GIF/1428651-89-1.gif)
1428651-89-1
0 suppliers
inquiry
![4-BROMO-5H-CYCLOPENTA[C]PYRIDIN-7(6H)-ONE(WXC07919)](/CAS/20180906/GIF/1428651-90-4.gif)
1428651-90-4
29 suppliers
inquiry
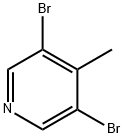
3430-23-7
208 suppliers
$5.00/250mg
![4-BROMO-5H-CYCLOPENTA[C]PYRIDIN-7(6H)-ONE(WXC07919)](/CAS/20180906/GIF/1428651-90-4.gif)
1428651-90-4
29 suppliers
inquiry
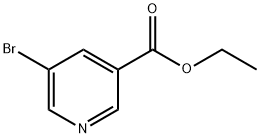
20986-40-7
270 suppliers
$6.00/10g
![4-BROMO-5H-CYCLOPENTA[C]PYRIDIN-7(6H)-ONE(WXC07919)](/CAS/20180906/GIF/1428651-90-4.gif)
1428651-90-4
29 suppliers
inquiry