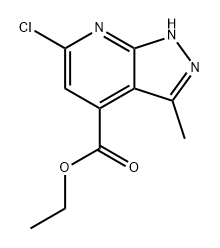
ethyl 6-chloro-3-methyl-1H-pyrazolo[3,4-b]pyridine-4-carboxylate synthesis
- Product Name:ethyl 6-chloro-3-methyl-1H-pyrazolo[3,4-b]pyridine-4-carboxylate
- CAS Number:1429171-52-7
- Molecular formula:C10H10ClN3O2
- Molecular Weight:239.66
![Ethyl 3-methyl-6-oxo-6,7-dihydro-1H-pyrazolo[3,4-b]pyridine-4-carboxylate](/CAS/20180629/GIF/1018166-61-4.gif)
1018166-61-4
10 suppliers
inquiry
![ethyl 6-chloro-3-methyl-1H-pyrazolo[3,4-b]pyridine-4-carboxylate](/CAS/20180703/GIF/1429171-52-7.gif)
1429171-52-7
20 suppliers
$277.00/250mg
Yield:1429171-52-7 88%
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene;trichlorophosphate in toluene at 0 - 110; for 5 h;
Steps:
1.b (b) 6-Chloro-3-methyl-1 H-pyrazol -b]pyridine-4-carboxylic acid ethyl ester
(b) 6-Chloro-3-methyl-1 H-pyrazol -b]pyridine-4-carboxylic acid ethyl ester6-Hydroxy-3-methyl-1 H-pyrazolo[3,4-b]pyridine-4-carboxylic acid ethyl ester (30.0 g), phosphorous oxychloride (12.6 mL) and 1 ,8-diazabicyclo[5.4.0]undec-7-ene (22.3 mL) were dissolved in toluene (550 mL) at 0°C (ice bath), then stirred at 1 10°C for 5 h, then cooled to r.t.. The mixture was poured into a vigorously stirred solution of potassium acetate (39.9 g) in water (1 I). The aqueous solution was extracted with ethyl acetate, the organic layer was dried over magnesium sulfate and concentrated in vacuo. The crude product was used for the next step without further purification. 28.6 g (88%) of the title compound were obtained.1H-NMR (500MHz, d6-DMSO): 1 .39 (t, 3H), 2.60 (s, 3H), 4.44 (q, 2H), 7.53 (s, 1 H), 13.70 (s, 1 H).LC/MS (Method LC1 ): Rt = 1 .19 min; m/z = 240.1 [M+H]+.
References:
WO2013/45413,2013,A1 Location in patent:Page/Page column 311