
[4-(4-FLUORO-BENZYL)-4-HYDROXY-PIPERIDIN-1-YL]-(2-PYRIMIDIN-4-YL-PYRIDIN-3-YL)-METHANONE synthesis
- Product Name:[4-(4-FLUORO-BENZYL)-4-HYDROXY-PIPERIDIN-1-YL]-(2-PYRIMIDIN-4-YL-PYRIDIN-3-YL)-METHANONE
- CAS Number:1429505-54-3
- Molecular formula:C22H21FN4O2
- Molecular Weight:392.43

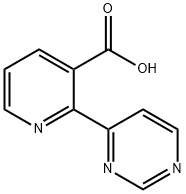
1429505-77-0
13 suppliers
$285.00/100mg
1429555-05-4
10 suppliers
$351.00/1g
![[4-(4-FLUORO-BENZYL)-4-HYDROXY-PIPERIDIN-1-YL]-(2-PYRIMIDIN-4-YL-PYRIDIN-3-YL)-METHANONE](/CAS/20180601/GIF/1429505-54-3.gif)
1429505-54-3
6 suppliers
inquiry
Yield:1429505-54-3 100%
Reaction Conditions:
Stage #1: 2-(pyrimidin-4-yl)nicotinic acidwith N-ethyl-N,N-diisopropylamine;O-(7-azabenzotriazol-1-yl)-n,n,n',n'-tetramethyluronium hexafluoro-phosphate in N,N-dimethyl-formamide at 0; for 0.333333 h;
Stage #2: 4-(4-fluorobenzyl)piperidin-4-ol hydrochloride in N,N-dimethyl-formamide at 25; for 0.5 h;
Steps:
3.3 Step 3
To a solution of 2-(pyrimidin-4-yl)nicotinic acid (200 mg, 0.994 mmol) in DMF (10 mL) was added HATU (566 mg, 1.49 mmol) at 0oC. To this mixture was added DIPEA (385 mg, 2.98 mmol) dropwise. After stirring at 0oC for 20 minutes, 4-[(4-fluorophenyl)methyl]piperidin-4-ol hydrochloride (244 mg, 0.99 mmol) was added. The reaction was warmed to 25oC and stirred for 30 minutes. The reaction mixture was concentrated. A second reaction was performed in parallel and the crude reaction mixtures were combined for purification. The combined reactions were purified by prep-HPLC (column: Xamide 150*30mm 5μm, gradient: 15-45% B (A= water, 10mM NH4HCO3, B= ACN), flow rate: 25 mL/min) to give (4-(4-fluorobenzyl)-4-hydroxypiperidin-1-yl)(2-(pyrimidin-4-yl)pyridin-3-yl)methanone (325 mg, 100%).1H-NMR (400 MHz, CDCl3) δH 9.19 (s, 0.5H), 8.94 (s, 0.5H), 8.90-8.81 (m, 1H), 8.76-8.72 (m, 1H), 8.29-8.18 (m, 1H), 7.75-7.60 (m, 1H), 7.52-7.38 (m, 1H), 7.18-7.12 (m, 2H), 7.05-6.98 (m, 2H), 4.70-4.46 (m, 1H), 3.64-2.88 (m, 4H), 2.77 (s, 2H), 1.95-1.61 (m, 2H), 1.41-1.24 (m, 1H), 1.21 (s, 1H). LC-ELSD/MS purity 100%, ESI cal. for C22H22FN4O2[M +H]+393, found 393.
References:
WO2022/115620,2022,A1 Location in patent:Paragraph 0271; 0276-0277

4763-58-0
14 suppliers
inquiry
![[4-(4-FLUORO-BENZYL)-4-HYDROXY-PIPERIDIN-1-YL]-(2-PYRIMIDIN-4-YL-PYRIDIN-3-YL)-METHANONE](/CAS/20180601/GIF/1429505-54-3.gif)
1429505-54-3
6 suppliers
inquiry

1429505-88-3
0 suppliers
inquiry
![[4-(4-FLUORO-BENZYL)-4-HYDROXY-PIPERIDIN-1-YL]-(2-PYRIMIDIN-4-YL-PYRIDIN-3-YL)-METHANONE](/CAS/20180601/GIF/1429505-54-3.gif)
1429505-54-3
6 suppliers
inquiry

1256794-15-6
9 suppliers
inquiry
![[4-(4-FLUORO-BENZYL)-4-HYDROXY-PIPERIDIN-1-YL]-(2-PYRIMIDIN-4-YL-PYRIDIN-3-YL)-METHANONE](/CAS/20180601/GIF/1429505-54-3.gif)
1429505-54-3
6 suppliers
inquiry