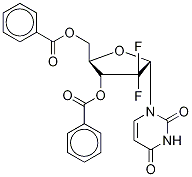
1'-Epi 2',2'-Difluoro-2'-deoxyuridine 3',5'-Dibenzoate synthesis
- Product Name:1'-Epi 2',2'-Difluoro-2'-deoxyuridine 3',5'-Dibenzoate
- CAS Number:143157-24-8
- Molecular formula:C23H18F2N2O7
- Molecular Weight:472.39
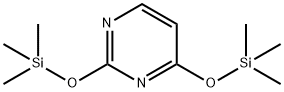
890044-84-5
0 suppliers
inquiry
10457-14-4
74 suppliers
$65.00/10mg

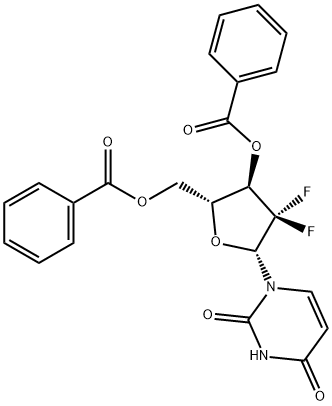
143157-24-8
13 suppliers
$149.90/10mg
143157-27-1
29 suppliers
$70.00/10mg
Yield:95 % de
Reaction Conditions:
with tin(IV) chloride in 1,2-dichloro-ethane at 20 - 83; for 22 h;Product distribution / selectivity;
Steps:
2
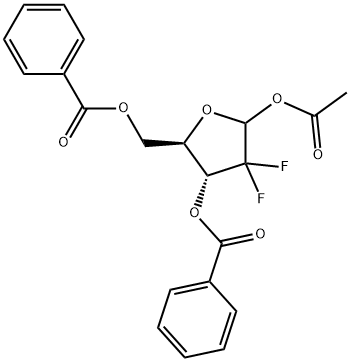
A 0.46 g sample of l-acetyl-2-deoxy-3,5-dibenzoate-2,2-difluoro-ribofuranose(compound IV that can be obtained from commercially available material e.g. by method EPO
References:
WO2006/63105,2006,A1 Location in patent:Page/Page column 11-12
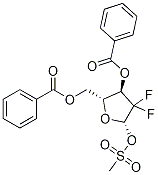
134877-42-2
126 suppliers
$45.00/25mg

10457-14-4
74 suppliers
$65.00/10mg

143157-24-8
13 suppliers
$149.90/10mg

143157-27-1
29 suppliers
$70.00/10mg