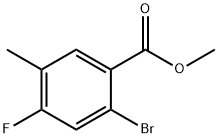
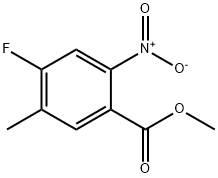
2-Bromo-4-fluoro-5-methyl-benzoic acid methyl ester synthesis
- Product Name:2-Bromo-4-fluoro-5-methyl-benzoic acid methyl ester
- CAS Number:1437780-13-6
- Molecular formula:C9H8BrFO2
- Molecular Weight:247.06
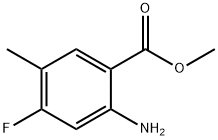
1037206-86-2
25 suppliers
$65.00/10mg

1437780-13-6
11 suppliers
inquiry
Yield:1437780-13-6 74%
Reaction Conditions:
Stage #1: methyl 2-amino-4-fluoro-5-methylbenzoatewith hydrogen bromide;sodium nitrite in water at 0; for 0.5 h;
Stage #2: with copper(I) bromide in water at 0 - 20; for 1 h;
Steps:
1.41.4 Step 4: Preparation of methyl 2-bromo-4-fluoro-5-methylbenzoate
Step 4:
Preparation of methyl 2-bromo-4-fluoro-5-methylbenzoate
To the suspension of methyl 2-amino-4-fluoro-5-methylbenzoate (2.2 g, 12 mmol) in 48% hydrobromic acid (80 mL) was added an aqueous solution (32 mL) of sodium nitrite (828 mg, 12 mmol) at 0° C., and the resulting solution was stirred for 30 min.
Copper (I) bromide (1.72 g, 12 mmol) was added to the solution at 0° C. and the resulting reaction mixture was stirred at r.t for 1 h.
After addition of ethyl acetate (200 mL), the reaction mixture was washed with water (200 mL*2), dried over sodium sulfate, filtered and concentrated under reduced pressure.
The residue was purified by column chromatography on silica gel eluted with PE-EA (10:1) to give methyl 2-bromo-4-fluoro-5-methylbenzoate (2.2 g, yield 74%) as colorless oil. 1H NMR (500 MHz, CDCl3) δ 7.655 (d, J=8.0 Hz, 1H), 7.257 (d, J=8.5 Hz, 1H), 3.837 (s, 3H), 2.177 (s, 3H) ppm.
References:
US2013/131016,2013,A1 Location in patent:Paragraph 0431; 0432
403-15-6
194 suppliers
$5.00/1g

1437780-13-6
11 suppliers
inquiry
1437780-11-4
8 suppliers
inquiry

1437780-13-6
11 suppliers
inquiry
1437780-12-5
9 suppliers
inquiry

1437780-13-6
11 suppliers
inquiry