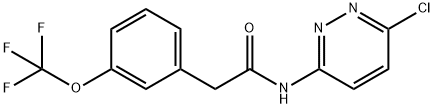
N-(6-chloropyridazin-3-yl)-2-(3-(trifluoromethoxy)phenyl)acetamide synthesis
- Product Name:N-(6-chloropyridazin-3-yl)-2-(3-(trifluoromethoxy)phenyl)acetamide
- CAS Number:1439400-46-0
- Molecular formula:C13H9ClF3N3O2
- Molecular Weight:331.68
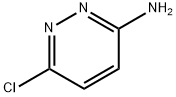
5469-69-2
377 suppliers
$6.00/10g
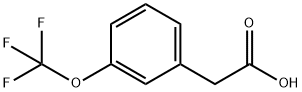
203302-97-0
168 suppliers
$6.00/250mg

1439400-46-0
14 suppliers
inquiry
Yield:1439400-46-0 85%
Reaction Conditions:
Stage #1: 6-chloro-pyridazin-3-ylamine;2-(3-(trifluoromethoxy)phenyl)acetic acidwith N-ethyl-N,N-diisopropylamine in ethyl acetate; for 0.25 h;Inert atmosphere;
Stage #2: with 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide in ethyl acetate at 20.3 - 28.1; for 0.666667 h;
Steps:
1
A three liter reaction vessel was charged with 2-(3-(trifluoromethoxy)phenyl)acetic acid(93.34 g, 0.43 mol, 1.0 equiv), 6-chloropyridazin-3-amine (55.51 g, 0.43 mol, 1.0 equiv.),ethyl acetate (1.42 L, 15 vol. versus acid), N,N-diisopropylethylamine (60.92 g, 0.47 mol, 1.1 equiv.) then fitted with a stir bar and temperature probe. The contents of the reaction vessel was placed under an atmosphere of argon(g) and stirred for 15 minutes at which time the mixture was turbid with solids on the bottom of the reaction vessel. To the stirredmixture was added propylphosphonic anhydride (T3P; 300 mL of 50% solution in ethyl acetate, 0.47 mmol, 1.1 equiv.) via a pressure equalizing addition funnel over the course of 40 minutes with a temperature increase of 20.3°C to 28.1°C. During the course of the addition the color of the mixture became red/orange and the turbidity cleared. The reaction was monitored by TLC (6:4 hexane / ethyl acetate) with a typical run time of 4-6 hours.When the reaction was deemed complete, water (1.5 L) was added and the mixture stirred for an additional 15 minutes. The mixture was transferred to a separatory funnel and the layers separated. The organic layer was washed with water (1.5 L) the layers separated and the organic layer washed with 10% sodium chloride solution (500 mL). The layers were separated, the organic layer transferred to a round bottom flask and the volatiles removedunder reduced pressure to give an off-white, yellow solid. To the flask was added hexanes (500 mL) and the contents stirred vigorously for 15 minutes then filtered. The solids were washed again with hexanes (500 mL) and air dried to a constant weight to afford N-(6- chloropyridazin-3 -yl)-2-(3 -(trifluoromethoxy)phenyl)acetamide (112824): yield of 121.1 g, (85%). ‘H NMR (300 MHz, DMSO-d6) ? 11.63 (s, 1H), 8.38(d, J=9.4 Hz, 1H), 7.88(d,J9.4 Hz, 1H), 7.52 - 7.27(m, 4H), 3.90(s, 2H).
References:
WO2016/22969,2016,A1 Location in patent:Page/Page column 39