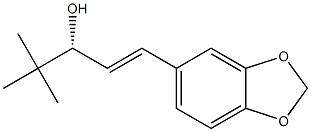
(E,3R)-1-(1,3-benzodioxol-5-yl)-4,4-dimethylpent-1-en-3-ol synthesis
- Product Name:(E,3R)-1-(1,3-benzodioxol-5-yl)-4,4-dimethylpent-1-en-3-ol
- CAS Number:144017-65-2
- Molecular formula:C14H18O3
- Molecular Weight:234.2909
Yield:144017-65-2 15%
Reaction Conditions:
with tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride in dichloromethane;Inert atmosphere;Heating;Enzymatic reaction;Alkene (Olefin) Metathesis;
Steps:
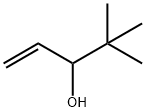
4.7. Synthesis of (R)-(+)-1-(benzo[d][1,3]dioxol-5-yl)-4,4-dimethylpent-1-en-3-ol (R)-(+)-4, (R)-(+)-Stiripentol
To a stirred solution of 5-vinylbenzo[d][1,3]dioxole 1 (22 mg, 0.15 mmol) and (R)-(+)-4,4-dimethylpent-1-en-3-ol (R)-(+)-2 (17 mg, 0.15 mmol) in dry DCM (5 mL), Grubbs’ second generation catalyst (7 mg, 5 mol %) was added and the mixture was heated at overnight under argon, the reaction mixture was cooled, and the product purified using preparative thin layer chromatography (silica gel and CHCl3) to afford 5 mg of (R)-(+)-Stiripentol (15%, ee >99%). The enantiomeric excess was calculated according to the reported procedures by Jacobsen et al.
References:
El-Behairy, Mohammed Farrag;Sundby, Eirik [Tetrahedron Asymmetry,2013,vol. 24,# 5-6,p. 285 - 289]
331-39-5
732 suppliers
$5.00/100mg

144017-65-2
6 suppliers
inquiry
630-19-3
328 suppliers
$13.00/1g

144017-65-2
6 suppliers
inquiry
6053-02-7
55 suppliers
$260.00/10mg

144017-65-2
6 suppliers
inquiry
![5-ethenylbenzo[1,3]dioxole](/CAS/GIF/7315-32-4.gif)