
Benzoic acid, 4-[1-[(2,4-diamino-6-pteridinyl)methyl]-3-butyn-1-yl]-, sodium salt synthesis
- Product Name:Benzoic acid, 4-[1-[(2,4-diamino-6-pteridinyl)methyl]-3-butyn-1-yl]-, sodium salt
- CAS Number:1445586-50-4
- Molecular formula:C18H15N6NaO2
- Molecular Weight:370.3405
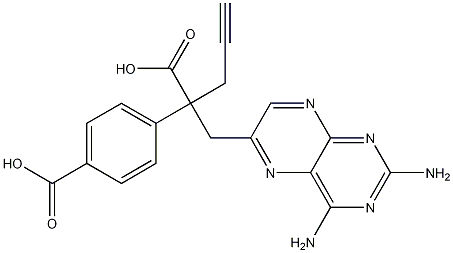
146464-92-8
31 suppliers
$505.42/5MG
![Benzoic acid, 4-[1-[(2,4-diamino-6-pteridinyl)methyl]-3-butyn-1-yl]-, sodium salt](/CAS/20180527/GIF/1445586-50-4.gif)
1445586-50-4
12 suppliers
inquiry
Yield:1445586-50-4 50.3%
Reaction Conditions:
Stage #1: 4-(2-carboxy-1-(2,4-diaminopteridin-6-yl)pent-4-yn-2-yl)benzoic acid in dimethyl sulfoxide at 110 - 115;
Stage #2: with water;sodium hydride in isopropyl alcohol at 50 - 55;
Steps:
8 10-Propargyl-4-deoxy-4-amino-10-deazapteroic Acid sodium salt
A solution of 10-Propargyl-10-carboxy-4-deoxy-4-amino-10-deazapteroic Acid (40 g) in DMSO (400 mL) was added to preheated DMSO (1400 mL) at 110-115° C and stirred for 20-30 min. The reaction was monitored by HPLC and upon completion; the reaction mixture was concentrated to dryness under vacuum below 90 °C. IPA (600 mL) was added to the residue and heated to 50-55 °C for 2-3 hours, subsequently cooled to 20-25 °C and vacuum filtered the solid. For preparing sodium salt of the acid the solid obtained was added to DM water (100 mL), followed by drop wise addition of aqueous NaOH solution (6.11 g NaOH in 300 mL DM water) at 10-15° C. The reaction mass was further cooled to 0-5 °C and stirred for 2- 3 hours. The solid was filtered under vacuum. The solid obtained was purified by dissolving in a mixture of DM water (320 mL) and IPA (640 mL) at 75-85 °C. The clear solution was filtered through Celite bed (20 g). The filtrate was cooled slowly to 0-5 °C and stirred for 1-2 hours. The solid obtained was filtered and dried under vacuum at 60-65 °C to give 19 g (50.3 %) of the title compound. Purity: 99.3 %.
References:
WO2014/16740,2014,A2 Location in patent:Page/Page column 24
![Benzoic acid, 4-[1-[(2,4-diamino-6-pteridinyl)methyl]-3-butyn-1-yl]-](/CAS2/GIF/146464-93-9.gif)
146464-93-9
45 suppliers
inquiry
![Benzoic acid, 4-[1-[(2,4-diamino-6-pteridinyl)methyl]-3-butyn-1-yl]-, sodium salt](/CAS/20180527/GIF/1445586-50-4.gif)
1445586-50-4
12 suppliers
inquiry
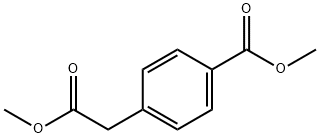
52787-14-1
140 suppliers
$12.00/1g
![Benzoic acid, 4-[1-[(2,4-diamino-6-pteridinyl)methyl]-3-butyn-1-yl]-, sodium salt](/CAS/20180527/GIF/1445586-50-4.gif)
1445586-50-4
12 suppliers
inquiry
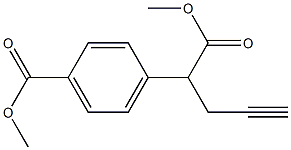
146464-90-6
58 suppliers
inquiry
![Benzoic acid, 4-[1-[(2,4-diamino-6-pteridinyl)methyl]-3-butyn-1-yl]-, sodium salt](/CAS/20180527/GIF/1445586-50-4.gif)
1445586-50-4
12 suppliers
inquiry
![6-Pteridinepropanoic acid, 2,4-diaMino-α-[4-(Methoxycarbonyl)phenyl]-α-2-propyn-1-yl-, Methyl ester](/CAS/20150408/GIF/146464-91-7.gif)
146464-91-7
15 suppliers
inquiry
![Benzoic acid, 4-[1-[(2,4-diamino-6-pteridinyl)methyl]-3-butyn-1-yl]-, sodium salt](/CAS/20180527/GIF/1445586-50-4.gif)
1445586-50-4
12 suppliers
inquiry