
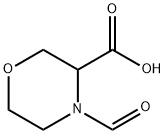
1H-Pyrrolo[2,1-c][1,4]oxazine-8-carboxylic acid, 3,4-dihydro- synthesis
- Product Name:1H-Pyrrolo[2,1-c][1,4]oxazine-8-carboxylic acid, 3,4-dihydro-
- CAS Number:1449136-89-3
- Molecular formula:C8H9NO3
- Molecular Weight:167.16
![1H-Pyrrolo[2,1-c][1,4]oxazine-8-carboxylic acid, 3,4-dihydro-, ethyl ester](/CAS/20210305/GIF/1449136-33-7.gif)
1449136-33-7
0 suppliers
inquiry
![1H-Pyrrolo[2,1-c][1,4]oxazine-8-carboxylic acid, 3,4-dihydro-](/CAS/20200515/GIF/1449136-89-3.gif)
1449136-89-3
2 suppliers
inquiry
Yield:1449136-89-3 100%
Reaction Conditions:
with water;sodium hydroxide in methanol; for 4 h;Reflux;
Steps:
3,4-Dihydro-1H-pyrrolo[2,1-c][1,4]oxazine-8-carboxylic acid (comp. no.8a)
To a solution of 3,4-dihydro-1H-pyrrolo[2,1-c][1 ,4]oxazine-8-carboxylic acid ethyl ester (comp. no. 2a) (3.2 g, 16.4 mmol) in methanol (64 ml) was added water (64 ml) and sodium hydroxide (3.3 g, 82 mmol) and the mixture was stirred at reflux until complete conversion according to LC/MC (about 4 h). Approximately half of the solvents were evaporated in vacuo and the mixture was acidified with excess hydrochloric acid. The forming precipitate was filtered off, washed once with water and dried in vacuo to give 2.7 g (100 %) of 3,4-dihydro-1H-pyrrolo[2,1-c][1,4]oxazine-8-carboxylic acid as white solid. LC/MS (method 4): Rt = 0.77 min; m/z = 168.02 (M+H+).
References:
WO2013/113860,2013,A1 Location in patent:Page/Page column 131; 132
1449136-32-6
4 suppliers
inquiry
![1H-Pyrrolo[2,1-c][1,4]oxazine-8-carboxylic acid, 3,4-dihydro-](/CAS/20200515/GIF/1449136-89-3.gif)
1449136-89-3
2 suppliers
inquiry