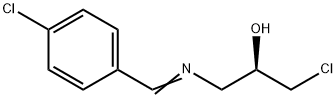
(R)-1-chloro-3-{[(4-chlorophenyl)methylene]amino}propan-2-ol synthesis
- Product Name:(R)-1-chloro-3-{[(4-chlorophenyl)methylene]amino}propan-2-ol
- CAS Number:1450915-93-1
- Molecular formula:C10H11Cl2NO
- Molecular Weight:232.11
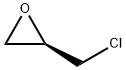
51594-55-9
375 suppliers
$10.00/1g
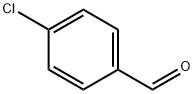
104-88-1
646 suppliers
$5.00/25g
![(R)-1-chloro-3-{[(4-chlorophenyl)methylene]amino}propan-2-ol](/CAS/20180601/GIF/1450915-93-1.gif)
1450915-93-1
11 suppliers
inquiry
Yield:1450915-93-1 51%
Reaction Conditions:
Stage #1: 4-chlorobenzaldehydewith ammonia in tetrahydrofuran;water; for 0.5 h;
Stage #2: (R)-(-)-epichlorohydrin in tetrahydrofuran;water at 20 - 45; for 24 h;
Steps:
4 (preparation of (R)-1-chloro-3-{[(4-chlorophenyl)methylene]amino}propan-2-ol
55 ml of an aqueous solution of ammonia (25-28%) were added to a solution of 4- chlorobenzaldehyde (75 g) in 250 ml of tetrahydrofuran. The mixture was stirred at the laboratory temperature for ca. 30 minutes. Then, 41.5 ml of (i?)-epichlorohydrin and 50 ml of tetrahydrofuran were added. The mixture was stirred at ca. 20°C for 19 hours and then at 40- 45 °C for 4 hours and then being slowly cooled to a temperature below 30°C for another 1 hour. After that 200 ml of toluene was added. The separated organic layer was washed with water (2x50 ml) and dried over sodium sulphate. After removal of the desiccant by filtration the filtrate was concentrated in vacuo, 50 ml of toluene added to the residue and the mixture was again concentrated in vacuo. 400 ml of heptane and 40 ml of toluene were added to the obtained oil, the emulsion was stirred and heated at 60-65 °C until a solution was produced. The solution was stirred under very slow cooling from the temperature of ca. 60°C to the target temperature of the suspension of ca. 25°C for 1.5 hours. The separated crystalline product was filtered, washed with heptane (2x50 ml) and dried. 64.2 g (yield 51%) of off- white crystals with the melt point of 69-70°C were obtained, GC 99.4%, content of the (S isomer 0.09% according to GC. 1H NMR (250 MHz, DMSO-D6), δ (ppm): 3.61 (m, 2H); 3.73 (m, 2H); 3.95 (m, 1H); 5.26 (m, 1H); 7.51 (m, 2H); 7.77 (m, 2H); 8.34 (s, 1H). 13C NMR (250 MHz, DMSO-D6), δ (ppm): 47.9; 63.5; 69.9, 128.7; 129.5; 134.8; 135.2; 161,3. MS (m/z): 232.0289 (M+H)+.
References:
WO2013/120465,2013,A1 Location in patent:Page/Page column 21; 22