
tert-butyl3-amino-4-(hydroxymethyl)-3-(isothiazol-5-yl)pyrrolidine-1-carboxylate synthesis
- Product Name:tert-butyl3-amino-4-(hydroxymethyl)-3-(isothiazol-5-yl)pyrrolidine-1-carboxylate
- CAS Number:1463484-93-6
- Molecular formula:C13H21N3O3S
- Molecular Weight:299.39
![tert-butyl 6a-isothiazol-5-yl-3,3a,4,6-tetrahydro-1H-pyrrolo[3,4-c]isoxazole-5-carboxylate](/CAS/20180703/GIF/1463484-63-0.gif)
1463484-63-0
3 suppliers
inquiry

1463484-93-6
4 suppliers
inquiry
Yield:1463484-93-6 65.1%
Reaction Conditions:
with acetic acid;zinc at 40; for 8 h;
Steps:
73.c tert-Butyl 3-amino-4-(hydroxymethyl)-3-isothiazol-5-yl-pyrrolidine-1-carboxylate
Preparation 73c
tert-Butyl 3-amino-4-(hydroxymethyl)-3-isothiazol-5-yl-pyrrolidine-1-carboxylate
tert-Butyl 6a-isothiazol-5-yl-3,3a,4,6-tetrahydro-1H-pyrrolo[3,4-c]isoxazole-5-carboxylate (400.0 g, 1.35 mol), acetic acid (4.00 L) and zinc (439.78 g, 6.73 mol) are added together.
The reaction mixture is heated to 40° C. and stirred at 40° C. for 8 hours and then cooled to room temperature.
The reaction mixture is diluted with ethyl acetate (4 L), filtered through diatomaceous earth, washed with ethyl acetate, and evaporated under reduced pressure.
The yellow oily residue is dissolved in toluene (2 L) and concentrated.
The dissolution process with toluene is repeated 3 times.
The foamy oil residue is suspended in 10% w/w aq. citric acid (3.2 L), MTBE (4 L) is added and the mixture is stirred for 15 minutes at room temperature.
The biphasic mixture is filtered through diatomaceous earth (slow filtration) to remove the gel-like solids.
The layers are separated and the aqueous layer is washed with MTBE (4*600 mL).
The aqueous layer (pH 4.0) is added to ethyl acetate (3.0 L), and the mixture is neutralized with NaOH 50% w/w to adjust the pH=9.0-9.5 and the mixture is vigorously stirred.
The organic layer is separated and the aqueous layer is extracted with ethyl acetate (3*1 L).
The organic layers are combined, dried over sodium sulfate, filtered, and evaporated under reduced pressure to give the title compound as a white solid, (262.00 g, 65.1%). ES/MS (m/e): 300 (M+H).
References:
US2013/261111,2013,A1 Location in patent:Paragraph 0186; 0187
aMino}acetic acid](/CAS/20150408/GIF/145618-68-4.gif)
145618-68-4
19 suppliers
$65.00/50mg

1463484-93-6
4 suppliers
inquiry
![tert-butyl N-allyl-N-[2-(methoxy(methyl)amino)-2-oxo-ethyl]carbamate](/CAS/20180702/GIF/1429646-79-6.gif)
1429646-79-6
3 suppliers
inquiry

1463484-93-6
4 suppliers
inquiry

1463484-37-8
3 suppliers
inquiry

1463484-93-6
4 suppliers
inquiry
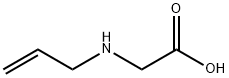
3182-77-2
23 suppliers
inquiry

1463484-93-6
4 suppliers
inquiry