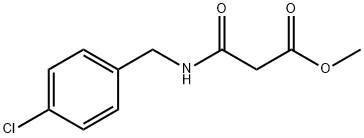
Propanoic acid, 3-[[(4-chlorophenyl)methyl]amino]-3-oxo-, methyl ester synthesis
- Product Name:Propanoic acid, 3-[[(4-chlorophenyl)methyl]amino]-3-oxo-, methyl ester
- CAS Number:1466514-73-7
- Molecular formula:C11H12ClNO3
- Molecular Weight:241.67
Yield:1466514-73-7 81.3%
Reaction Conditions:
with triethylamine in dichloromethane at -78; for 0.666667 h;
Steps:
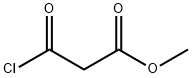
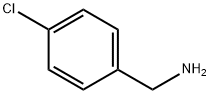
1 Preparation of methyl 3-{[(4-chlorophenyl)methyl]amino}-3-oxopropanoate, (3a).
To a solution of 4-chlorobenzylamine (1a) (7.8 mL, 57.5 mmol) in DCM was added Et3N (24 mL, 172.6 mmol) and the mixture was cooled in a -78 °C bath. A solution of methyl 3- chloro-3-oxopropionate (2a) (7.0 mL, 57.5 mmol) in DCM (20 mL) was added via a dropping funnel over 10 min. The mixture was stirred at -78 °C for 30 min and then it was slowly warmed to room temperature. After overnight, the reaction was quenched with saturated NaHCO3 solution. The layers were separated and the organic layer was washed with brine, dried over Na2SO4, filtered and concentrated under reduced pressure. The solid was suspended in ~10 mL EtOAc and further diluted with 200 mL hexanes. The resulting precipitate was filtered, washed with hexanes providing (3a) as a light yellow solid (11.3 g, 81.3% yield).
References:
WO2021/174355,2021,A1 Location in patent:Page/Page column 86-88