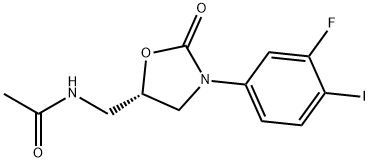
(S)-N-[3-(3-FLUORO-4-IODO-PHENYL)-2-OXO-OXAZOLIDIN-5-YLMETHYL]-ACETAMIDE synthesis
- Product Name:(S)-N-[3-(3-FLUORO-4-IODO-PHENYL)-2-OXO-OXAZOLIDIN-5-YLMETHYL]-ACETAMIDE
- CAS Number:149524-45-8
- Molecular formula:C12H12FIN2O3
- Molecular Weight:378.14
![(S)-N-[[3-(3-Fluorophenyl)-2-oxo-5-oxazolidinyl]methyl]acetamide](/CAS/GIF/139071-79-7.gif)
139071-79-7
44 suppliers
$110.00/50mg
![(S)-N-[3-(3-FLUORO-4-IODO-PHENYL)-2-OXO-OXAZOLIDIN-5-YLMETHYL]-ACETAMIDE](/CAS/GIF/149524-45-8.gif)
149524-45-8
33 suppliers
$208.00/250mg
Yield: 92%
Reaction Conditions:
with N-iodo-succinimide;trifluoroacetic acid at 20; for 1 h;Inert atmosphere;
Steps:
(S)-N-((3-(3-fluoro-4-iodophenyl)-2-oxooxazolidin-5-yl)methyl)acetamide (7).
To asolution of (S)-N-((3-(3-fluorophenyl)-2-oxooxazolidin-5-yl)methyl)acetamide (6) (2.9g, 1 equiv) in TFA (12 mL, 1M) was added NIS (2.72 g, 1.05 equiv) portion-wise. Thereaction was stirred for 1 h at rt. While stirring, the exothermic reaction turned pinked.Next, the TFA was concentrated off under reduced pressure. The crude mixture was dilutedwith Na2S2O3 (10%), and then KOH was added until pH = 14. Next the solution was dilutedto ~700 mL volume with H2O. Extraction with CH2Cl2 (4x50 mL) and EtOAc (4x50 mL)was followed by drying with Na2SO4 and concentration of the combined organic layersunder reduced pressure. The crude product was then purified by silica gel flashchromatography (5 → 10% MeOH/CH2Cl2) to afford the desired iodinated compound 7 (4g, 92% yield). Product identity matched previously reported characterization data.4
References:
Sun, Alexander W.;Bulterys, Philip L.;Bartberger, Michael D.;Jorth, Peter A.;O'Boyle, Brendan M.;Virgil, Scott C.;Miller, Jeff F.;Stoltz, Brian M. [Bioorganic and Medicinal Chemistry Letters,2019,vol. 29,# 18,p. 2686 - 2689] Location in patent:supporting information
627543-03-7
6 suppliers
inquiry

108-24-7
0 suppliers
$14.00/250ML
![(S)-N-[3-(3-FLUORO-4-IODO-PHENYL)-2-OXO-OXAZOLIDIN-5-YLMETHYL]-ACETAMIDE](/CAS/GIF/149524-45-8.gif)
149524-45-8
33 suppliers
$208.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![AcetaMide, N-[(2S)-2-(acetyloxy)-3-chloropropyl]-](/CAS/GIF/183905-31-9.gif)
183905-31-9
205 suppliers
$45.00/5g
![(S)-N-[3-(3-FLUORO-4-IODO-PHENYL)-2-OXO-OXAZOLIDIN-5-YLMETHYL]-ACETAMIDE](/CAS/GIF/149524-45-8.gif)
149524-45-8
33 suppliers
$208.00/250mg
519003-01-1
0 suppliers
inquiry
507-09-5
328 suppliers
$12.00/5g
![(S)-N-[3-(3-FLUORO-4-IODO-PHENYL)-2-OXO-OXAZOLIDIN-5-YLMETHYL]-ACETAMIDE](/CAS/GIF/149524-45-8.gif)
149524-45-8
33 suppliers
$208.00/250mg
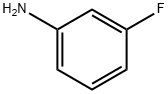
372-19-0
320 suppliers
$10.00/1g
![(S)-N-[3-(3-FLUORO-4-IODO-PHENYL)-2-OXO-OXAZOLIDIN-5-YLMETHYL]-ACETAMIDE](/CAS/GIF/149524-45-8.gif)
149524-45-8
33 suppliers
$208.00/250mg