2,2-DIMETHYL-N-[2-METHYL-3-(TRIFLUOROMETHYL)PHENYL]-PROPIONAMIDE synthesis
- Product Name:2,2-DIMETHYL-N-[2-METHYL-3-(TRIFLUOROMETHYL)PHENYL]-PROPIONAMIDE
- CAS Number:150783-50-9
- Molecular formula:C13H16F3NO
- Molecular Weight:259.27
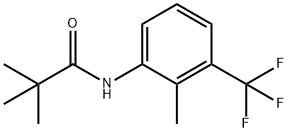
1939-19-1
34 suppliers
inquiry

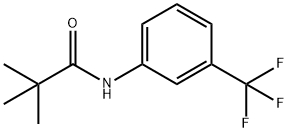
74-88-4
356 suppliers
$15.00/10g
![2,2-DIMETHYL-N-[2-METHYL-3-(TRIFLUOROMETHYL)PHENYL]-PROPIONAMIDE](/CAS/GIF/150783-50-9.gif)
150783-50-9
20 suppliers
$130.00/100mg
Yield:-
Reaction Conditions:
with n-butyllithium in tetrahydrofuran;hexane;
Steps:
Preparation of 2,2,2'-Trimethyl-3'-Trifluoromethyl-Propionanilide
Preparation of 2,2,2'-Trimethyl-3'-Trifluoromethyl-Propionanilide A solution of 2,2-dimethyl-3'-trifluoromethyl-propionanilide (6.1 g, 25 mmol) in 100 ml dry tetrahydrofuran was cooled to 0° C. under nitrogen atmosphere and a solution of n-BuLi (1.6M in hexane, 50 ml, 75 mmol) was added dropwise in such a rate that the internal temperature did not rise above 10° C. (about 20 min.) and it was stirred at that temperature for 10 more minutes during which time a grayish turbidity appeared. The cooling bath was removed and the reaction mixture was stirred at room temperature for 2.5 hours. It was cooled to about 5° C. again and a solution of methyl iodide (4.258 g, 30 mmol) in tetrahydrofuran (10 ml.) was slowly transferred via a canula. It was stirred at room temperature for 4 hours and then quenched with water. The solution was concentrated in vacuo and then diluted with water and acidified with dilute HCl. It was then extracted with ether, and the ethereal extracts were washed with water followed by brine. The ether extract was dried with MgSO4 and concentrated to give a light yellow creamy solid (6.2 g., 95%). This was purified by crystallization from dichloromethane and petroleum ether mixture to afford colorless needles of 2,2,2'-trimethyl-3'-trifluoromethyl-propionanilide (80-85%, m.p. 122.5° C.).
References:
US5248781,1993,A
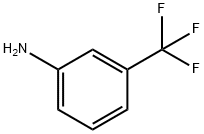
98-16-8
396 suppliers
$9.00/5g
![2,2-DIMETHYL-N-[2-METHYL-3-(TRIFLUOROMETHYL)PHENYL]-PROPIONAMIDE](/CAS/GIF/150783-50-9.gif)
150783-50-9
20 suppliers
$130.00/100mg