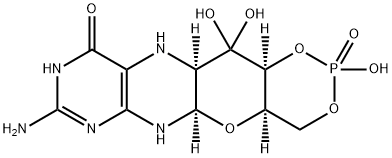
(4aR,5aR,11aR,12aS)-8-amino-2,12,12-trihydroxy-4a,5a,6,9,11,11a,12,12a-octahydro-[1,3,2]dioxaphosphinino[4',5':5,6]pyrano[3,2-g]pteridin-10(4H)-one2-oxide synthesis
- Product Name:(4aR,5aR,11aR,12aS)-8-amino-2,12,12-trihydroxy-4a,5a,6,9,11,11a,12,12a-octahydro-[1,3,2]dioxaphosphinino[4',5':5,6]pyrano[3,2-g]pteridin-10(4H)-one2-oxide
- CAS Number:150829-29-1
- Molecular formula:C10H14N5O8P
- Molecular Weight:363.22
![4H-Pyrano[3,2-g]pteridin-4-one, 2-amino-3,5,5a,6,7,8,9a,10-octahydro-6,7-dihydroxy-8-(hydroxymethyl)-, (5aS,6R,7R,8R,9aR)-](/CAS/20210305/GIF/1394061-94-9.gif)
1394061-94-9
0 suppliers
inquiry
![(4aR,5aR,11aR,12aS)-8-amino-2,12,12-trihydroxy-4a,5a,6,9,11,11a,12,12a-octahydro-[1,3,2]dioxaphosphinino[4',5':5,6]pyrano[3,2-g]pteridin-10(4H)-one2-oxide](/CAS/20210111/GIF/150829-29-1.gif)
150829-29-1
7 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: (5aS,6R,7R,8R,9aR)-2-amino-6,7-dihydroxy-8-(hydroxymethyl)-3H,4H,5H,5aH,6H,7H,8H,9aH,10H-pyrano[3,2-g]pteridin-4-onewith dmap;di-tert-butyl dicarbonate in tetrahydrofuran at 50; for 20 h;Inert atmosphere;
Stage #2: with sodium methylate in methanol;
Steps:
1.B; 1.C; 1.D; 1.E; 1.F
B. Preparation of compounds 2 (a or b) and 3 (a, b or c).; Di-tert-butyl dicarbonate (10.33 g, 47.3 mmol) and DMAP (0.321 g, 2.63 mmol) were added to a stirred suspension of 1 (1.5 g, 5.26 mmol) in anhydrous THF (90 mL) at 50 °C under Ar. After 20 h a clear solution resulted. The solvent was evaporated and the residuechromatographed on silica gel (gradient of 0 to 40 % EtOAc in hexanes) to give two product fractions. The first product to elute was a yellow foam (1.46 g). The product was observed to be a mixture of two compounds by NMR containing mainly a product with seven Boc groups (2a or 2b). A sample was crystallized from EtOAc-hexanes to give 2a or 2b as a fine crystalline solid. MPt 189 - 191 °C. [a] *° -^3.6 (c 0.99, MeOH). 1H NMR (500 MHz, CDC13): δ 5.71 (t, J = 1.7 Hz, 1H), 5.15 (dt, 7= 3.5, - 1.0, 1H), 4.97 (t, J= 3.8, 1H), 4.35 (br t, J= ~ 1.7, 1H), 4.09- 3.97 (m, 3H), 3.91 (m, 1H), 1.55, 1.52, 1.51, 1.50, 1.45 (5s, 45H), 1.40 (s, 18H). 13C NMR (125.7 MHz, CDC13): δ 152.84 (C), 152.78 (C), 151.5 (C), 150.9 (C), 150.7 (2 χ C), 150.3 (C), 149.1 (C), 144.8 (C), 144.7 (C), 118.0 (C), 84.6 (C), 83.6 (C), 83.5 (C), 82.7 (3 χ C), 82.6 (C), 76.3 (CH), 73.0 (CH), 71.4 (CH), 67.2 (CH), 64.0 (C?), 51.4 (CH), 28.1 (CH3), 27.8 (2 x CH3), 27.7 (CH3), 27.6 (3 x CH3). MS-ESI+ for C45H72N5019+, (M+H)+, Calcd. 986.4817 found986.4818. Anal, calcd. for C45H7iN50i9 H20 54.39 C, 7.39 H, 6.34 N; found 54.66 C, 7.17 H, 7.05 N. A second fraction was obtained as a yellow foam (2.68 g) which by 1H NMR was a product with six Boc groups present (3a, 3b or 3c). A small amount was crystallized from EtOAc-hexanes to give colorless crystals, [a] 2D° -47.6 (c, 1.17, CHC13). 1H NMR (500 MHz, CDC13): δ 1 1.10 (br s, exchanged D20, 1H), 5.58 (t, J= 1.8 Hz, 1H), 5.17 (d, J= 3.4 Hz, 1H), 4.97 (t, J= 3.9 Hz, 1H), 4.62 (s, exchanged D20, 1H), 4.16 (dd, J= 11.3, 5.9 Hz, 1H), 4.12 (dd, 7= 11.3, 6.4 Hz, 1H), 3.95 (dt, J= 6.1, 1.1 Hz, 1H), 3.76 (m, 1H), 1.51, 1.50, 1.49, 1.48, 1.46 (5s, 54H). 13C NMR (125.7 MHz, CDC13): δ 156.6 (C), 153.0 (C), 152.9 (C), 151.9 (C), 150.6 (C), 149.4 (2 x C), 136.2 (C), 131.8 (C), 1 16.9 (C), 85.0 (2 x C), 83.3 (C), 82.8 (C), 82.49 (C), 82.46 (C), 73.3 (CH), 71.5 (CH), 67.2 (CH), 64.5 (CH2), 51.3 (CH), 28.0, 27.72, 27.68, 27.6 (4 x CH3). MS-ESI+ for C40H64N5Oi7+, (M+H)+ calcd. 886.4287, found 886.4289.; C. Preparation of compound 4a, 4b or 4c.; Step 1 - The first fraction from B above containing mainly compounds 2a or 2b (1.46 g, 1.481 mmol) was dissolved in MeOH (29 mL) and sodium methoxide in MeOH (1M, 8.14 mL, 8.14 mmol) added. After leaving at ambient temperature for 20 h the solution was neutralized with Dowex 50WX8 (H+) resin then the solids filtered off and the solvent evaporated.Step 2 - The second fraction from B above containing mainly 3a, 3b or 3c (2.68 g, 3.02 mmol) was dissolved in MeOH (54 mL) and sodium methoxide in MeOH (1M, 12.10 mL, 12.10 mmol) added. After leaving at ambient temperature for 20 h the solution was neutralized with Dowex 50WX8 (H+) resin then the solids filtered off and the solvent evaporated.The products from step 1 and step 2 above were combined and chromatographed on silica gel (gradient of 0 to 15 % MeOH in CHC13) to give 4a, 4b or 4c as a cream colored solid (1.97 g). 1H NMR (500 MHz, DMSO d6):6 12.67 (br s, exchanged D20, 1H), 5.48 (d, J= 5.2 Hz, exchanged D20, 1H), 5.43 (t, J= -1.9 Hz, after D20 exchange became a d, J= 1.9 Hz, 1H), 5.00 (br s, exchanged D20, 1H), 4.62 (d, J= 5.7 Hz, exchanged D20, 1H), 4.27 (d, J= 6.0 Hz, exchanged D20, 1), 3.89 (dt, J= 5.2, 3.8 Hz, after D20 became a t, J= 3.9 Hz, 1H), 3.62 (dd, J = 6.0, 3.7 Hz, after D20 exchange became a d, J= 3.7 Hz, 1H), 3.52 - 3.39 (m, 4H), 1.42 (s, 9H), 1.41 (s, 18H). 13C NMR (125.7 MHz, DMSO ?): δ 157.9 (C), 151.1, (C), 149.8 (2 χ C), 134.6 (C), 131.4 (C), 1 18.8 (C), 83.5 (2 χ C), 81.3 (C), 78.2 (CH), 76.5 (CH), 68.1 (CH), 66.8 (CH), 60.6 (CH2), 54.4 (CH), 27.9 (CH3), 27.6 (2 χ CH3). MS-ESI+ for C25H40 5O1 j+, (M+H)+ calcd. 586.2719, found 586.2717.; D. Preparation of compound 5a, 5b or 5c; Compound 4a, 4b or 4c (992 mg, 1.69 mmol) was dissolved in anhydrous pyridine and concentrated. The residue was dissolved in anhydrous CH2C12 (10 mL) and pyridine (5 mL) under a nitrogen atmosphere and the solution was cooled to -42 °C in an acetonitrile/dry ice bath. Methyl dichlorophosphate (187 μ, 1.86 mmol) was added dropwise and the mixture was stirred for 2 h 20 min. Water (10 mL) was added to the cold solution which was then removed from the cold bath and diluted with ethyl acetate (50 mL) and saturated NaCl solution (30 mL). The organic portion was separated and washed with saturated NaCl solution. The combined aqueous portions were extracted twice further with ethyl acetate and the combined organic portions were dried over MgS04 and concentrated. Purification by silica gel flash column chromatography (eluting with 2 - 20 % methanol in ethyl acetate) gave the cyclic methyl phosphate 5a, 5b or 5c (731 mg, 65%). 1H NMR (500 MHz, CDC13,):6 11.72 (bs, exchanged D20, 1H), 5.63 (t, J= 1.8 Hz, 1H), 5.41 (s, exchanged D20, 1H), 4.95 (d, J= 3.2 Hz, 1H), 4.70 (dt, J= 12.4, 1.8 Hz, 1H), 4.42 (dd, J= 22.1, 12.1 Hz, 1H). 4.15 (q, J= 3.7 Hz, 1H), 3.82 (s, 1H), 3.75 (s, 1H), 3.58 (d, J= 11.7 Hz, 3H), 2.10 (bs, exchanged D20, 1H + H20), 1.50 (s, 9H), 1.46 (s, 18H). 13C NMR (125.7 MHz, CDC13, centre line δ 77.0): δ 157.5 (C), 151.2 (C), 149.6 (2 x C), 134.5 (C), 132.3 (C), 117.6 (C), 84.7 (2 x C), 82.8 (C), 77.3 (CH), 74.8 (d, J= 4.1 Hz, CH), 69.7 (CH2), 68.8 (d, J= 4.1 Hz, CH), 68.6 (d, J= 5.9 Hz, CH), 56.0 (d, J= 7.4 Hz, CH3), 51.8 (CH), 28.1 (CH3), 27.8 (CH3). MS-ESI+ for C26H40N5NaOi3P+ (M+Na)+, calcd. 684.2252, found 684.2251.; E. Preparation of compound 6a, 6b or 6c; Compound 5a, 5b or 5c (223 mg, 0.34 mmol) was dissolved in anhydrous CH2C12 (7 mL) under a nitrogen atmosphere. Anhydrous DMSO (104 ., 1.46 mmol) was added and the solution was cooled to -78 °C. Trifluoroacetic anhydride (104 xL, 0.74 mmol) was added dropwise and the mixture was stirred for 40 min. N,N-diisopropylethylamine (513 μ,,2.94 mmol) was added and the stirring was continued for 50 min at -78 °C. Saturated NaCl solution (20 mL) was added and the mixture removed from the cold bath and diluted with CH2C12 (30 mL). Glacial acetic acid (170 iL, 8.75 mmol) was added and the mixture was stirred for 10 min. The layers were separated and the aqueous phase was washed with CH2C12 (10 mL). The combined organic phases were washed with 5 % aqueous HC1, 3 : 1 saturated NaCl solution: 10 % NaHC03 solution and saturated NaCl solution successively, dried over MgS04, and concentrated to give compound 6a, 6b or 6c (228 mg, quant.) of suitable purity for further use. NMR (500 MHz, CDC13): δ 5.86 (m, 1 H), 5.07 (m, 1 H), 4.70 - 4.64 (m, 2 H), 4.49 - 4.40 (m, 1 H), 4.27 (m, 1 H), 3.56, m, 4 H), 1.49 (s, 9 H), 1.46 (s, 18 H) ppm. 13C NMR (500 MHz, CDC13): δ 157.5 (C), 151.1 (C), 150.6 (2 C), 134.6 (C), 132.7 (C), 1 16.6 (C), 92.0 (C), 84.6 (2 C), 83.6 (C), 78.0 (CH), 76.0 (CH), 70.4 (CH2), 67.9 (CH), 56.2 (CH3) 56.0 (CH), 28.2 (3 CH3), 26.8 (6 CH3) ppm.31P NMR (500 MHz, CDC13): δ - 6.3 ppm. 9; F. Preparation of compound 7: (4aR,5aR, llaR, 12aS)-l, 3, 2-Dioxaphosphorinof 4 ', 5 ': 5, 6Jpyrano[3, 2-gJpteridin- 10(4H)-one, 8-amino- 4a,5a, 6, 9, 11, 11a, 12, 12a-octahydro-2, 12, 12-trihydroxy-2-oxide; Compound 6a, 6b or 6c (10 mg, 14.8 μηιο) was dissolved in dry acetonitrile (0.2 mL) and cooled to 0 °C. Bromotrimethylsilane (19.2 μ, 148 μηιοΓ) was added dropwise and the mixture was allowed to warm to ambient temperature and stirred for 5 h during which time a precipitate formed. HCl(aq) (10 μ, 37 %) was added and the mixture was stirred for a further 15 min. The mixture was centrifuged for 15 min (3000 g) and the resulting precipitate collected. Acetonitrile (0.5 mL) was added and the mixture was centrifuged for a further 15 min. The acetonitrile wash and centrifugation was repeated a further two times and the resulting solid was dried under high vacuum to give compound 7 (4 mg, 75 %). NMR (500 MHz, D20): δ 5.22 (d, J= 1.6 Hz, 1H), 4.34 (dt, J= 13, 1.6 Hz, 1H), 4.29 - 4.27 (m, 1H), 4.24 - 4.18 (m, lH), 3.94 (br m, 1H), 3.44 (t, J= 1.4 Hz, 1H). 31P NMR (500 MHz, D20): δ -4.8 MS-ESI+ forC10H15N5O8P+, (M+H)+ calcd. 364.0653, found 364.0652.
References:
WO2012/112922,2012,A1 Location in patent:Page/Page column 126-127; 129-132
![1,3,2-Dioxaphosphorino[4',5':5,6]pyrano[3,2-g]pteridine-6(5aH)-carboxylic acid, 8-[bis[(1,1-dimethylethoxy)carbonyl]amino]-4,4a,9,10,11,11a,12,12a-octahydro-12,12-dihydroxy-2-methoxy-10-oxo-, 1,1-dimethylethyl ester, 2-oxide, (4aR,5aR,11aR,12aS)-](/CAS/20210305/GIF/1394061-91-6.gif)
1394061-91-6
0 suppliers
inquiry
![(4aR,5aR,11aR,12aS)-8-amino-2,12,12-trihydroxy-4a,5a,6,9,11,11a,12,12a-octahydro-[1,3,2]dioxaphosphinino[4',5':5,6]pyrano[3,2-g]pteridin-10(4H)-one2-oxide](/CAS/20210111/GIF/150829-29-1.gif)
150829-29-1
7 suppliers
inquiry
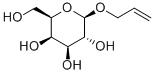
2595-07-5
75 suppliers
$45.00/100mg
![(4aR,5aR,11aR,12aS)-8-amino-2,12,12-trihydroxy-4a,5a,6,9,11,11a,12,12a-octahydro-[1,3,2]dioxaphosphinino[4',5':5,6]pyrano[3,2-g]pteridin-10(4H)-one2-oxide](/CAS/20210111/GIF/150829-29-1.gif)
150829-29-1
7 suppliers
inquiry
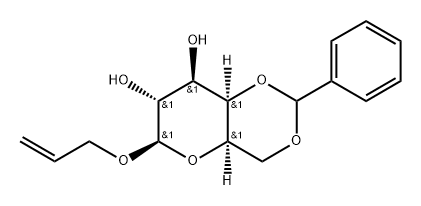
71925-95-6
3 suppliers
inquiry
![(4aR,5aR,11aR,12aS)-8-amino-2,12,12-trihydroxy-4a,5a,6,9,11,11a,12,12a-octahydro-[1,3,2]dioxaphosphinino[4',5':5,6]pyrano[3,2-g]pteridin-10(4H)-one2-oxide](/CAS/20210111/GIF/150829-29-1.gif)
150829-29-1
7 suppliers
inquiry

1394061-64-3
0 suppliers
inquiry
![(4aR,5aR,11aR,12aS)-8-amino-2,12,12-trihydroxy-4a,5a,6,9,11,11a,12,12a-octahydro-[1,3,2]dioxaphosphinino[4',5':5,6]pyrano[3,2-g]pteridin-10(4H)-one2-oxide](/CAS/20210111/GIF/150829-29-1.gif)
150829-29-1
7 suppliers
inquiry