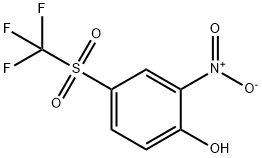
2-Hydroxy-5-[(trifluoromethyl)sulphonyl]nitrobenzene, 4-Hydroxy-3-nitrophenyl trifluoromethyl sulphone synthesis
- Product Name:2-Hydroxy-5-[(trifluoromethyl)sulphonyl]nitrobenzene, 4-Hydroxy-3-nitrophenyl trifluoromethyl sulphone
- CAS Number:15183-75-2
- Molecular formula:C7H4F3NO5S
- Molecular Weight:271.17
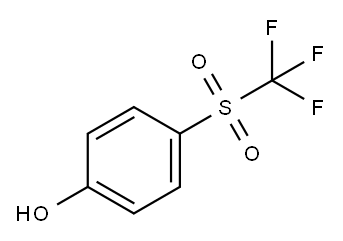
432-84-8
20 suppliers
$65.00/50mg
![2-Hydroxy-5-[(trifluoromethyl)sulphonyl]nitrobenzene, 4-Hydroxy-3-nitrophenyl trifluoromethyl sulphone](/CAS/GIF/15183-75-2.gif)
15183-75-2
19 suppliers
$136.00/250mg
Yield:15183-75-2 79.04 g
Reaction Conditions:
with sulfuric acid;nitric acid in water at 30; for 6 h;
Steps:
2 Example 2
To a mixture of 75.0 g of 4-(trifluoromethylsulfa- nyl)phenol, 37.5 g of water, 37.5 g of 2-ethylhexanoic acid and 6.38 g of sodium tungstate dihydrate, 112.60 g of 35% by weight of aqueous hydrogen peroxide was added drop- wise at 75° C. over 10.5 hours, and the mixture was stirred for 12 hours. A 22% by weight of aqueous sodium sulfite solution was added dropwise to the reaction mixture, and the layers were separated. The obtained aqueous layer was extracted with 37.5 g of 2-ethylhexanoic acid. The two obtained organic layers were combined, 7.5 g of water was added thereto, then 214.63 g of 96% by weight of sulfuric acid was added dropwise thereto, and 28.79 g of 98% by weight of nitric acid was further added dropwise thereto at 30° C. over 5 hours. Afier stirring for 1 hour, the reaction mixture was brought to room temperature, 29.27 g of water was added thereto, followed by adding dropwise 120.01 g of a 28% by weight of aqueous sodium hydroxide solution, and the solution was separated. To the obtained organic layer, 73.18 g of water was added and the layers were separated to obtain 187.16 g (content: 52.0% by weight) of a solution containing 4-(trifluoromethylsulfonyl)-2-nitrophenol.To the obtained solution, 150.0 g of hexane was added and the solution was cooled to 0° C. and kept at this temperature for 3 hours to precipitate crystals. The precipitated crystals were filtered, and washed sequentially with water and hexane, and the obtained crystals were dried under reduced pressure to obtain 79.04 g of 4-(trifluoromethylsul- fonyl)-2-nitrophenol (content: 98.5% by weight).
References:
US2018/186732,2018,A1 Location in patent:Paragraph 0051; 0052; 0053; 0054; 0056
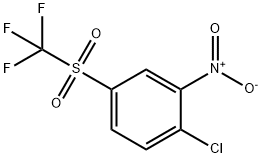
1550-27-2
33 suppliers
$144.00/1g
![2-Hydroxy-5-[(trifluoromethyl)sulphonyl]nitrobenzene, 4-Hydroxy-3-nitrophenyl trifluoromethyl sulphone](/CAS/GIF/15183-75-2.gif)
15183-75-2
19 suppliers
$136.00/250mg
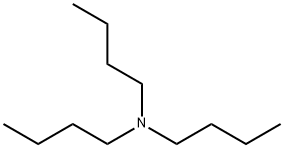
102-82-9
0 suppliers
$10.00/1ml

1550-27-2
33 suppliers
$144.00/1g
![2-Hydroxy-5-[(trifluoromethyl)sulphonyl]nitrobenzene, 4-Hydroxy-3-nitrophenyl trifluoromethyl sulphone](/CAS/GIF/15183-75-2.gif)
15183-75-2
19 suppliers
$136.00/250mg
6309-30-4
17 suppliers
inquiry
![Benzenamine, N,N-dibutyl-2-nitro-4-[(trifluoromethyl)sulfonyl]-](/CAS/20210305/GIF/75113-70-1.gif)
75113-70-1
0 suppliers
inquiry
![Benzene, 1-methoxy-2-nitro-4-[(trifluoromethyl)sulfonyl]-](/CAS/20200611/GIF/360-00-9.gif)
360-00-9
0 suppliers
inquiry
![2-Hydroxy-5-[(trifluoromethyl)sulphonyl]nitrobenzene, 4-Hydroxy-3-nitrophenyl trifluoromethyl sulphone](/CAS/GIF/15183-75-2.gif)
15183-75-2
19 suppliers
$136.00/250mg
461-84-7
267 suppliers
$16.00/5g
![2-Hydroxy-5-[(trifluoromethyl)sulphonyl]nitrobenzene, 4-Hydroxy-3-nitrophenyl trifluoromethyl sulphone](/CAS/GIF/15183-75-2.gif)
15183-75-2
19 suppliers
$136.00/250mg