
1-methyl-4-(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-1H-pyrazole synthesis
- Product Name:1-methyl-4-(3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-1H-pyrazole
- CAS Number:1534350-44-1
- Molecular formula:C16H21BN2O2
- Molecular Weight:284.16

141938-40-1
13 suppliers
inquiry

73183-34-3
587 suppliers
$6.00/5g

1534350-44-1
16 suppliers
inquiry
Yield:1534350-44-1 92%
Reaction Conditions:
Stage #1: 4-(3-bromo-phenyl)-1-methyl-1H-pyrazole;bis(pinacol)diboranewith potassium acetate in N,N-dimethyl-formamide; for 0.333333 h;
Stage #2: with 1,1'-bis-(diphenylphosphino)ferrocene;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in N,N-dimethyl-formamide at 80;
Steps:
1 Intermediate 1: 1 -methyl-4-[3-(4,4,5,5-tetramethyl-1 ,3,2-d ioxaborola n-2- yI)phenyl]-1 H-pyrazole
Intermediate 1: 1 -methyl-4-[3-(4,4,5,5-tetramethyl-1 ,3,2-d ioxaborola n-2- yI)phenyl]-1 H-pyrazole To a solution of 4-(3-Bromo-phenyl)-1-methyl-1H-pyrazole (Otava, 410 mg, 1.7mmol) in dry DMF (10 mL) was added bis(pinacolato)diborane (525 mg, 2.0mmol) and dried KOAc (340 mg, 3.4 mmol). The reaction mixture was degassedfor 20 mm before the addition of dppf (48 mg, 0.09 mmol) and(dppf)PdCI2.CH2CI2 (71 mg, 0.09 mmol). The reaction mixture was then heated at 80°C 0/N. It was filtered through celite and the filtrate was concentrated under reduced pressure to give the title compound as a yellow oil (550 mg, 92%). 1H NMR (300 MHz, DMSO-d6) ? 8.24-8.15 (s, 1 H), 7.89-7.82 (d, J 0.9 Hz, 1H),7.82-7.76 (t, J = 1.3 Hz, 1H), 7.73-7.64 (m, 1H), 7.53-7.45 (m, 1H), 7.42-7.29 (m, 1 H), 3.89 - 3.82 (s, 3H), 1.34 - 1.26 (s, 12H).
References:
WO2014/8992,2014,A1 Location in patent:Page/Page column 111; 112
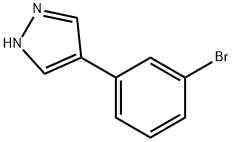
916792-28-4
29 suppliers
$65.00/500mg

1534350-44-1
16 suppliers
inquiry
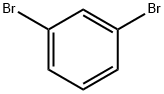
108-36-1
455 suppliers
$10.00/1g

1534350-44-1
16 suppliers
inquiry