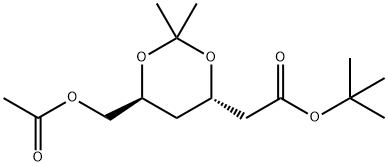
tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate synthesis
- Product Name:tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate
- CAS Number:154026-95-6
- Molecular formula:C15H26O6
- Molecular Weight:302.36
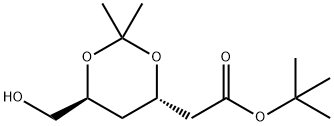
124655-09-0

75-36-5
![tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate](/CAS/GIF/154026-95-6.gif)
154026-95-6
Tert-butyl (4R,6S)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetate (1.0 g, 3.84 mmol) was dissolved in 10 mL of dichloromethane, and pyridine (0.9 g, 11.52 mmol) was added at room temperature and stirred for 30 min. Acetyl chloride (0.66 g, 8.45 mmol) was then added and stirring was continued at room temperature for 1 hour. After completion of the reaction, 10 mL of water was added to the mixture and the organic layer was separated by stirring for 10 minutes. The aqueous layer was extracted twice with 5 mL of dichloromethane, the organic layers were combined and washed with 10 mL of water to separate the organic layer. The organic layer was dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to afford 0.98 g (86% yield) of the target product (4R,6S)-tert-butyl (4R,6S)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate as light pink crystals.

124655-09-0
248 suppliers
$11.00/1g

75-36-5
589 suppliers
$17.92/100G
![tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate](/CAS/GIF/154026-95-6.gif)
154026-95-6
259 suppliers
$9.00/5g
Yield:154026-95-6 86%
Reaction Conditions:
Stage #1:1,1-dimethylethyl (4R-cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetate with pyridine in dichloromethane at 20; for 0.5 h;
Stage #2:acetyl chloride in dichloromethane at 20; for 1 h;
Steps:
3.1 Step 1: Preparation of the compound of formula I-5 {tert-butyl 2-((4R,6S)-6-(acetoxymethyl)-2,2-dimethyl-1,3-dioxan-4-yl)acetate}
The compound of formula (I-1){Tert-butyl 2 - ((4R, 6S) -6- (hydroxymethyl) -2,2-dimethyl-1,3-dioxan-4-yl) acetate}(1.0 g, 3.84 mmol, Jiangsu Alpha Pharmaceutical) was dissolved in 10 ml of methylene chloride (MC), pyridine (0.9 g, 11.52 mmol) was added at room temperature, and the mixture was stirred at room temperature for 30 minutes.Acetyl chloride (0.66 g, 8.45 mmol) was added and the mixture was stirred at room temperature for 1 hour. 10 ml of water was added to the reaction mixture, and the mixture was stirred for 10 minutes, and the organic layer was separated. After 5 ml of methylene chloride was extracted twice, 10 ml of water was added thereto, followed by stirringThe organic layer was separated. The organic layer was dried over anhydrous magnesium sulfate, filtered, and distilled under reduced pressure to obtain 0.98 g (yield: 86%) of the compound of the formula I-2 in the form of a pale pink crystal.
References:
Mirae Fine Chemical Co., Ltd.;Hwang, Sung-Kwan;Park, Sang-Hoo;Park, Chang-Ha;Yang, Joon-Ha KR2016/126700, 2016, A Location in patent:Paragraph 0161; 0162-0165
127-09-3
1177 suppliers
$8.00/1 ea
154026-94-5
128 suppliers
$115.00/25mg
![tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate](/CAS/GIF/154026-95-6.gif)
154026-95-6
259 suppliers
$9.00/5g
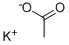
127-08-2
685 suppliers
$6.00/25g

154026-94-5
128 suppliers
$115.00/25mg
![tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate](/CAS/GIF/154026-95-6.gif)
154026-95-6
259 suppliers
$9.00/5g
10534-59-5
312 suppliers
$5.00/5g

154026-94-5
128 suppliers
$115.00/25mg
![tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate](/CAS/GIF/154026-95-6.gif)
154026-95-6
259 suppliers
$9.00/5g
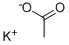
127-08-2
685 suppliers
$6.00/25g
143314-17-4
237 suppliers
$6.00/1g

154026-94-5
128 suppliers
$115.00/25mg
![tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate](/CAS/GIF/154026-95-6.gif)
154026-95-6
259 suppliers
$9.00/5g