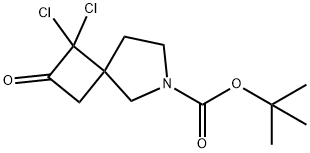
1,1-dichloro-2-oxo-6-Azaspiro[3.4]octane-6-carboxylic acid 1,1-dimethylethyl ester synthesis
- Product Name:1,1-dichloro-2-oxo-6-Azaspiro[3.4]octane-6-carboxylic acid 1,1-dimethylethyl ester
- CAS Number:1558037-99-2
- Molecular formula:C12H17Cl2NO3
- Molecular Weight:294.17
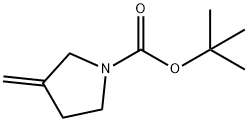
114214-71-0
113 suppliers
$31.00/250mg
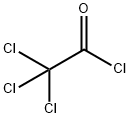
76-02-8
248 suppliers
$38.29/25G
![1,1-dichloro-2-oxo-6-Azaspiro[3.4]octane-6-carboxylic acid 1,1-dimethylethyl ester](/CAS/20180703/GIF/1558037-99-2.gif)
1558037-99-2
16 suppliers
inquiry
Yield:1558037-99-2 47%
Reaction Conditions:
with zinc/copper couple in 1,2-dimethoxyethane;diethyl ether at 10 - 20;Inert atmosphere;
Steps:
1-(Propylsulfonyl)dispiro[pyrrolidine-3,1'-cyclobutane-3',1''-[1,2]oxaborolo[4,3- d]pyrrolo[2,3-b]pyridin]-3''(6''H)-ol
The title compound was prepared by the scheme and procedures shown below: To a stirring solution of PPh3CH3Br (96.5 g, 270 mmol) in Et2O (1000 mL) was added t- BuOK (30.3 g, 270 mmol) and the mixture was stirred at rt for 1h before being treated with a solution of N-Boc-3-pyrrolidinone (50.0 g, 270 mmol) in Et2O (500 mL). The resulting mixture was stirred at room temperature overnight, and then it was poured into water and extracted with EtOAc. The organic layer was concentrated, and the residue was purified by column chromatography (PE/EtOAc = 100/1) to give the desired compound tert-butyl 3- methylenepyrrolidine-1-carboxylate (29.0 g, yield 58%) as a light oil.1H NMR (300 MHz, CDCl3): δ 4.96 (s, 2H), 3.91 (s, 2H), 3.45 (s, 2H), 2.66-2.42 (m, 2H), 1.46 (s, 9H) ppm. To a suspension of the obtained tert-butyl 3-methylenepyrrolidine-1-carboxylate (10.0 g, 54.6 mmol) in Et2O (75 ml) was added Zn-Cu (28.1 g, 21.8 mmol) at 10oC under N2 atmosphere. A solution of CCl3CCOCl (19.8 g, 109 mmol) in dimethoxyethane (DME, 75 mL) was added. The mixture was stirred at room temperature overnight, and then it was quenched with aqueous NaHCO3 and extracted with EtOAc. The combined organic phases were dried over sodium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica-gel chromatography to give tert-butyl 1,1-dichloro-2-oxo-6-azaspiro[3.4]octane-6-carboxylate (7.5 g, yield 47%) as a light-yellow oil.1H NMR (300 MHz, CDCl3): δ 4.00-3.80 (m, 1H), 3.75-3.29 (m, 4H), 3.29-3.12 (m, 1H), 2.57-2.39 (m, 1H), 2.02-1.88 (m, 1H), 1.48 (s, 9H) ppm. To a solution of tert-butyl 1,1-dichloro-2-oxo-6-azaspiro[3.4]octane-6-carboxylate (7.5 g, 25.6 mmol) in MeOH (80 mL) were added Zn (16.6 g, 256 mmol) and saturated NH4Cl (30 mL). The reaction mixture was stirred at room temperature for 3h. It was filtered and the filtrate was extracted with EtOAc. The combined organic phases were dried over sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography to give tert-butyl 2-oxo- 6-azaspiro[3.4]octane-6-carboxylate (5.2 g, yield 90%) as a white solid.1H NMR (400 MHz, CDCl3): δ 3.61-3.34 (m, 4H), 3.20-2.87 (m, 4H), 2.11-1.98 (m, 2H), 1.46 (s, 9H) ppm. MS: m/z =248.2 (M+Na)+. A solution of 5-bromo-4-iodo-1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridine (5.0 g, 10.5 mmol) in THF (100 mL) was added i-PrMgCl-LiCl (10.5 mL, 13.7 mmol) at -20oC under N2. The reaction was stirred at -20oC for 1h. To it was added slowly a solution of tert-butyl 2- oxo-6-azaspiro[3.4]octane-6-carboxylate (2.4 g, 10.5 mmol) in THF (50 mL). The reaction mixture was warmed to room temperature for 2h, then quenched with H2O and extracted with EtOAc. The organic layer was concentrated and the residue was purified by column chromatography (PE/EtOAc = 100/1 to 10/1) to give tert-butyl 2-(5-bromo-1-(triisopropylsilyl)- 1H-pyrrolo[2,3-b]pyridin-4-yl)-2-hydroxy-6-azaspiro[3.4]octane-6-carboxylate (1.0 g, yield 17%) as a white solid.1H NMR (400 MHz, CDCl3): δ 8.30 (s, 1H), 7.30 (s, 1H), 6.59 (d, J = 3.5 Hz, 1H), 3.81-3.61 (m, 2H), 3.45-3.25 (m, 2H), 2.96-2.71 (m, 4H), 1.86-1.78 (m, 5H), 1.48 (s, 9H), 1.10 (d, J = 6.8 Hz, 18H) ppm. MS: m/z =578.2 and 580.2 (M+H)+. To a solution of tert-butyl 2- (5-bromo-1-(triisopropylsilyl)-1H-pyrrolo[2,3-b]pyridin-4-yl)-2-hydroxy-6-azaspiro[3.4]octane-6- carboxylate (360 mg, 0.62 mmol) in THF (15 mL) were added (BPin)2 (304 mg, 1.2 mmol), KOAc (176 mg, 1.8 mmol) and Pd(dppf)Cl2 (196 mg, 0.24 mmol) under N2 atmosphere. The reaction was stirred at 75oC overnight, and then it was filtered, and the filtrate was concentrated. The residue was dissolved in THF (10 mL), and 6N HCl (2 mL) was added. The mixture was stirred for 30 min and extracted with EtOAc. The organic layer was concentrated to give a black solid (450 mg). It was mixed with DCM (5 mL) and TFA (1 mL), and the mixture was stirred for 2h. The solution was concentrated under reduce pressure to give the crude dispiro[pyrrolidine- 3,1'-cyclobutane-3',1''-[1,2]oxaborolo[4,3-d]pyrrolo[2,3-b]pyridin]-3''(6''H)-ol TFA salt (400 mg) as a black solid. To a solution of dispiro[pyrrolidine-3,1'-cyclobutane-3',1''-[1,2]oxaborolo[4,3- d]pyrrolo[2,3-b]pyridin]-3''(6''H)-ol TFA salt (400 mg, crude) in DCM (10 mL) were added Et3N (101 mg, 1.0 mmol) and propane-1-sulfonyl chloride (142 mg, 1.0 mmol) at 0oC. The reaction mixture was stirred at 0oC for 2h, and then it was poured to water and extracted with EtOAc. The organic layer was concentrated and the residue was purified by pre-HPLC (NH4OH in CH3CN and H2O) to give the desired product 1-(propylsulfonyl)dispiro[pyrrolidine-3,1'- cyclobutane-3',1''-[1,2]oxaborolo[4,3-d]pyrrolo[2,3-b]pyridin]-3''(6''H)-ol (12 mg, yield 5%) as a white solid.1H NMR (400 MHz, DMSO-d6): δ 12.01 (s, 1H), 9.29 (s, 1H), 8.49 (s, 1H), 7.61 (t, J = 2.8 Hz, 1H), 6.72 (dd, J = 3.4, 1.6 Hz, 1H), 3.63 (s, 2H), 3.40-3.35 (m, 2H), 3.16-3.03 (m, 2H), 2.87-2.83 (m, 2H), 2.34-2.30 (m, 2H), 2.20 (t, J = 7.0 Hz, 2H), 1.76-1.70 (m, 2H), 1.01 (t, J = 7.4 Hz, 3H) ppm. HPLC purity: 95.60 % at 210 nm. MS: m/z =376.1 (M+H)+.
References:
WO2021/61823,2021,A1 Location in patent:Paragraph 0110-0112