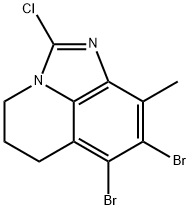
7,8-Dibromo-2-chloro-9-methyl-5,6-dihydro-4H-imidazo[4,5,1-ij]quinoline synthesis
- Product Name:7,8-Dibromo-2-chloro-9-methyl-5,6-dihydro-4H-imidazo[4,5,1-ij]quinoline
- CAS Number:1609452-31-4
- Molecular formula:C11H9Br2ClN2
- Molecular Weight:364.4636
![2-CHLORO-9-METHYL-5,6-DIHYDRO-4H-IMIDAZO[4,5,1-IJ]QUINOLINE](/CAS/20180601/GIF/1609453-56-6.gif)
1609453-56-6
![7,8-Dibromo-2-chloro-9-methyl-5,6-dihydro-4H-imidazo[4,5,1-ij]quinoline](/CAS/20180713/GIF/1609452-31-4.gif)
1609452-31-4
7,8-Dibromo-2-chloro-9-methyl-5,6-dihydro-4H-imidazo[4,5,1-IJ]quinoline was synthesized as follows (Method 2A): 2-chloro-9-methyl-5,6-dihydro-4H-imidazo[4,5,1-IJ]quinoline (11.7 g, 56 mmol, Method 14A) was dissolved in acetonitrile (250 mL) pre-cooled to 0 °C in acetonitrile (250 mL). Subsequently, N-bromosuccinimide (50.0 g, 282 mmol) was added in batches over 1 hour. The reaction mixture was continued to be stirred at 0 °C for 30 min, after which it was brought to room temperature and stirred for 48 hours. After completion of the reaction, the solvent was removed by distillation under reduced pressure. The residue was dissolved in dichloromethane and washed with saturated aqueous sodium bicarbonate. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated the filtrate to afford 18.6 g of the target product 7,8-dibromo-2-chloro-9-methyl-5,6-dihydro-4H-imidazo[4,5,1-IJ]quinoline in 90% yield. The product was characterized by LC-MS (m/z 364.8 [M + 1]) and 1H NMR (600 MHz, DMSO) δ 4.12 (t, 2H, CH2), 2.88 (t, 2H, CH2), 2.57 (s, 3H, CH3), 2.22-2.18 (m, 2H, CH2).
![2-CHLORO-9-METHYL-5,6-DIHYDRO-4H-IMIDAZO[4,5,1-IJ]QUINOLINE](/CAS/20180601/GIF/1609453-56-6.gif)
1609453-56-6
3 suppliers
inquiry
![7,8-Dibromo-2-chloro-9-methyl-5,6-dihydro-4H-imidazo[4,5,1-ij]quinoline](/CAS/20180713/GIF/1609452-31-4.gif)
1609452-31-4
19 suppliers
inquiry
Yield:1609452-31-4 90%
Reaction Conditions:
with N-Bromosuccinimide in acetonitrile at 0 - 20; for 49.5 h;
Steps:
7, 8-dibromo-2-chloro-9-methyl-5 ,6-dihydro-4H-imidazo [4,5,1 -]quino line (Method2A)
7, 8-dibromo-2-chloro-9-methyl-5 ,6-dihydro-4H-imidazo [4,5,1 -]quino line (Method2A) A 2-chloro-9-methyl-5,6-dihydro-4H-imidazo[4,5,1-]quinoline (11.7 g, 56 mmol)(Method 14A) was dissolved in acetonitrile (250 mL) cooled to 0°C and next Nbromosuccinimide (50.0 g, 282 mmol) was added portionwise over lh. The reaction mixture was stirred at 0°C for 30minutes then at room temperature for 48 hours. The solvent was evaporated under reduced pressure and residue was dissolved in dichloromethane and washed with saturated aqueous solution of sodium hydrogencarbonate. The organic layer was dried over anhydrous sodium sulfate, filtered and the filtrate was concentrated to gave 18.6g of 7,8-dibromo-2-chloro-9-methyl-5,6- dihydro-4H-imidazo[4,5,1-]quinoline; yield 90%; LC-MS (m/z) 364.8 (M+1). 1H NMR (600 MHz, DMSO) ? 4.12 (t, 2H, CH2), 2,88 (t, 2H, CH2), 2.57 (s, 3H, CH3), 2.22-2.18 (m, 2H, CH2).
References:
WO2014/72435,2014,A1 Location in patent:Page/Page column 79

160431-49-2
24 suppliers
$145.00/100mg
![7,8-Dibromo-2-chloro-9-methyl-5,6-dihydro-4H-imidazo[4,5,1-ij]quinoline](/CAS/20180713/GIF/1609452-31-4.gif)
1609452-31-4
19 suppliers
inquiry
![4H-Imidazo[4,5,1-ij]quinolin-2(1H)-one,5,6-dihydro-9-methyl-(9CI)](/CAS/20180808/GIF/36182-77-1.gif)
36182-77-1
8 suppliers
inquiry
![7,8-Dibromo-2-chloro-9-methyl-5,6-dihydro-4H-imidazo[4,5,1-ij]quinoline](/CAS/20180713/GIF/1609452-31-4.gif)
1609452-31-4
19 suppliers
inquiry