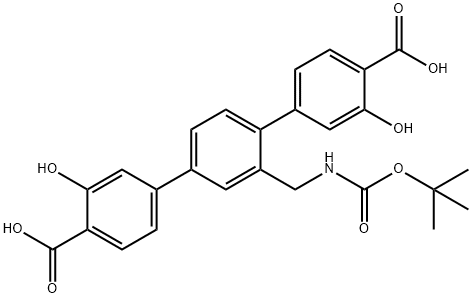
[1,1':4',1''-Terphenyl]-4,4''-dicarboxylic acid, 2'-[[[(1,1-dimethylethoxy)carbonyl]amino]methyl]-3,3''-dihydroxy- synthesis
- Product Name:[1,1':4',1''-Terphenyl]-4,4''-dicarboxylic acid, 2'-[[[(1,1-dimethylethoxy)carbonyl]amino]methyl]-3,3''-dihydroxy-
- CAS Number:1613451-83-4
- Molecular formula:C26H25NO8
- Molecular Weight:479.48
![[1,1':4',1''-Terphenyl]-4,4''-dicarboxylic acid, 2'-[[[(1,1-dimethylethoxy)carbonyl]amino]methyl]-3,3''-dihydroxy-, 4,4''-dimethyl ester](/CAS/20210305/GIF/1613451-79-8.gif)
1613451-79-8
0 suppliers
inquiry
![[1,1':4',1''-Terphenyl]-4,4''-dicarboxylic acid, 2'-[[[(1,1-dimethylethoxy)carbonyl]amino]methyl]-3,3''-dihydroxy-](/CAS/20200611/GIF/1613451-83-4.gif)
1613451-83-4
10 suppliers
inquiry
Yield:1613451-83-4 95%
Reaction Conditions:
Stage #1: C28H29NO8with water;sodium hydroxide in tetrahydrofuran at 50;
Stage #2: with hydrogenchloride in water at 20;
Steps:
2 Scheme 8. Synthetic path for organic linker 5c:
General procedure: General procedure 2 (saponification). To a solution of the required ester (1.0 relative mol amount) in THF was added a solution of NaOH (10 relative mol amount) in H20 to obtain a final concentration of 0.2 M for the ester (equal volumes of 0 and THF) . The resulting solution was stirred vigorously at 50 °C until the reaction completes (progress periodically checked by TLC) . After cooled down to room temperature, THF was removed under vacuum to give typically slightly yellow solution. While being stirred, the aqueous layer was acidified with dilute HC1 (1-3 M) and the resulting precipitate was collected by vacuum filtration, washed with ample H20, and dried in air for 24 h then in vacuo for 6 h to provide the corresponding hydrolysed product as typically a white powder.
Scheme 8. Synthetic path for organic linker 5c:
Compound 4c (2.10 g, 4.15 mmol) was hydrolyzed as described above to provide linker 5c as an off-white powder (1.88 g, 3.92 mmol, 95%). 1H NMR (500 MHz, DMSO-d6), [ppm]: 1.20 and 1.37 (two s, 9H), 4.16 (d, J= 5.9 Hz, 2H), 6.94 (d, J = 8.3 Hz, 1H), 6.96 (s, 1H), 7.25 and 7.27 (overlapping s and d, 2H), 7.33 (d, J = 8.0 Hz, 1H), 7.49 (t, J= 5.9 Hz, 1H), 7.68 (d, J = 7.9 Hz, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.90 (d, J = 8.7 Hz, 1H); 13C{1H} NMR (126 MHz, DMSO-d6), [ppm]: 28.3, 41.3, 78.0, 112.1, 112.3, 114.7, 117.5, 117.6, 120.3, 125.4, 126.2, 130.1, 130.3, 131.1, 137.7, 138.3, 139.5, 146.7, 147.2, 155.8, 161.0, 161.6, 171.9; IR (ATR) v-max [cm-1]: 3400-2700 (br w), 2976 (w), 2931 (w), 2871 (w), 2567 (w), 1659 (s), 1619 (s), 1563 (m), 1500 (m), 1480 (m), 1454 (m), 1434 (m), 1394 (w), 1366 (m), 1340 (m), 1251 (s), 1202 (s), 1158 (s), 1097 (m), 1046 (w), 1027 (w), 905 (m), 875 (m), 844 (w), 816 (m), 778 (s), 692 (m), 595 (w), 569 (w), 510 (w), 456 (w); MS (HR-ESI), m/z calcd. for C26H24NO8 [M-H]- 478.1502, found 478.1499.
References:
WO2015/195179,2015,A2 Location in patent:Paragraph 00154; 00172; 00174
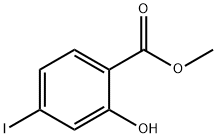
18179-39-0
141 suppliers
$8.00/1g
![[1,1':4',1''-Terphenyl]-4,4''-dicarboxylic acid, 2'-[[[(1,1-dimethylethoxy)carbonyl]amino]methyl]-3,3''-dihydroxy-](/CAS/20200611/GIF/1613451-83-4.gif)
1613451-83-4
10 suppliers
inquiry
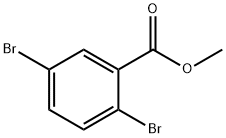
57381-43-8
122 suppliers
$9.00/1g
![[1,1':4',1''-Terphenyl]-4,4''-dicarboxylic acid, 2'-[[[(1,1-dimethylethoxy)carbonyl]amino]methyl]-3,3''-dihydroxy-](/CAS/20200611/GIF/1613451-83-4.gif)
1613451-83-4
10 suppliers
inquiry
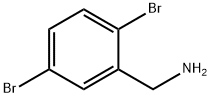
1214331-41-5
13 suppliers
inquiry
![[1,1':4',1''-Terphenyl]-4,4''-dicarboxylic acid, 2'-[[[(1,1-dimethylethoxy)carbonyl]amino]methyl]-3,3''-dihydroxy-](/CAS/20200611/GIF/1613451-83-4.gif)
1613451-83-4
10 suppliers
inquiry