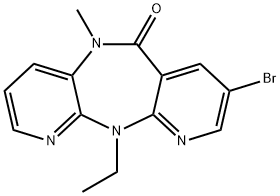
6H-Dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one,8-bromo-11-ethyl-5,11-dihydro-5-methyl- synthesis
- Product Name:6H-Dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one,8-bromo-11-ethyl-5,11-dihydro-5-methyl-
- CAS Number:162109-00-4
- Molecular formula:C14H13BrN4O
- Molecular Weight:333.18
Yield:162109-00-4 99%
Reaction Conditions:
Stage #1: 5,11-dihydro-11-ethyl-8-bromo-6H-dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-onewith sodium hydride in DMF (N,N-dimethyl-formamide) at 50; for 0.5 h;
Stage #2: methyl iodide at 20; for 1.5 h;
Steps:
To a solution of the 8-bromo-5,11-dihydro-11-ethyl-6H-dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one 1e (36.7 g, 115 mmol) in DMF (380 mL) was added NaH (3.5 g, 138 mmol), and the mixture was heated to 50° C. for 30 min. The reaction mixture was cooled to room temperature and treated with MeI (14.3 mL, 230 mmol). After 1.5 h, the reaction mixture was poured over ice water. The solid was filtered, washed with water and then hexane to give after drying, compound 1f (37.9 g, 99% yield) as a dark grey solid.
References:
US2004/106791,2004,A1 Location in patent:Page 9
![8-BROMO-11-ETHYL-5,11-DIHYDRO-6H-DIPYRIDO[3,2-B:2',3'-E][1,4]DIAZEPIN-6-ONE](/CAS/GIF/134698-42-3.gif)
134698-42-3
5 suppliers
inquiry
![6H-Dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one,8-bromo-11-ethyl-5,11-dihydro-5-methyl-](/CAS/GIF/162109-00-4.gif)
162109-00-4
4 suppliers
inquiry
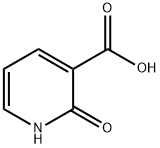
609-71-2
371 suppliers
$5.00/5g
![6H-Dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one,8-bromo-11-ethyl-5,11-dihydro-5-methyl-](/CAS/GIF/162109-00-4.gif)
162109-00-4
4 suppliers
inquiry
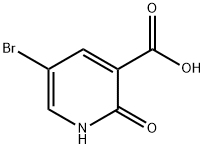
104612-36-4
242 suppliers
$8.00/1g
![6H-Dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one,8-bromo-11-ethyl-5,11-dihydro-5-methyl-](/CAS/GIF/162109-00-4.gif)
162109-00-4
4 suppliers
inquiry
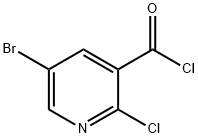
78686-86-9
23 suppliers
$106.04/250mgs:
![6H-Dipyrido[3,2-b:2',3'-e][1,4]diazepin-6-one,8-bromo-11-ethyl-5,11-dihydro-5-methyl-](/CAS/GIF/162109-00-4.gif)
162109-00-4
4 suppliers
inquiry
