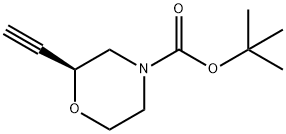
4-Morpholinecarboxylic acid, 2-ethynyl-, 1,1-dimethylethyl ester, (2S)- synthesis
- Product Name:4-Morpholinecarboxylic acid, 2-ethynyl-, 1,1-dimethylethyl ester, (2S)-
- CAS Number:1621165-19-2
- Molecular formula:C11H17NO3
- Molecular Weight:211.26
913642-85-0
56 suppliers
$135.00/100MG
90965-06-3
208 suppliers
$30.25/1g

1621165-19-2
15 suppliers
inquiry
Yield: 64%
Reaction Conditions:
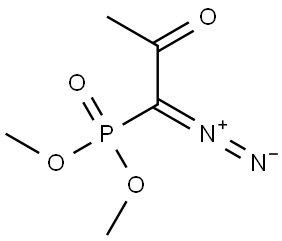
Stage #1:dimethyl 1-(1-diazo-2-oxopropyl)phosphonate with potassium carbonate in methanol;acetonitrile at 18 - 20; for 0.333333 h;
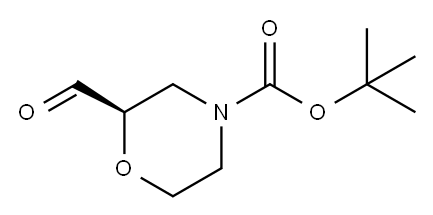
Stage #2:(R)-2-formylmorpholin-4-carboxylic acid tert-butyl ester in methanol;acetonitrile at 18 - 23;
Steps:
7.D j219j Part D. Preparation of (S)-tert-butyl 2-ethynylmorpholine-4- carboxylate
To a solution of dimethyl (1-diazo-2-oxopropyl)phosphonate (64.7 g, 0.337 mol) in a mixture of acetonitrile (526 mL) and methanol (105 mL) was added potassium carbonate (80.9 g, 0.585 mol). The suspension was stirred at 18-20 °C for 15 mm. A solution of tertbutyl (2R)-2-formylmorpholine-4-carboxylate (63.0 g, 0.293 mol) in a mixture ofacetonitrile (53 mL) and methanol (10 mL) was then added drop wise over 1 hr while maintaining the temperature between 18 and 23 °C. The resulting mixture was stirred for 2 hr at 20 °C after the end of the addition and was then held overnight. The resulting suspension was filtered and the filtrate was evaporated under reduced pressure. The resulting oil was added slowly to water (950 mL), and the precipitate was collected byfiltration, and the filter cake was washed with water (120 mL). The residue was purifiedby chromatography on silica gel (eluting with 10 % ethyl acetate in heptanes) to give (8)-tert-butyl 2-ethynylmorpholine-4-carboxylate (45.25 g, 64 % yield) as a solid. ‘H NMR(400 MHz, CDC13) 6 ppm 1.47 (s, 9 H), 2.49 (s, 1 H), 3.20 - 3.32 (m, 2 H), 3.49 - 3.61 (m,2 H), 3.70 - 3.90 (m, 1 H), 3.90 - 3.99 (m, 1 H), 4.21 - 4.28 (m, 1 H); chiral HPLC(Chiracel OB-H 0.46 x 250 mm, hexanes 93 % - ethanol 3 %, 30°C, 0.6 mL/min, isocratic30 mm) 97.0 % desired enantiomer.
References:
NEOMED INSTITUTE;BUON, Christophe;CANTIN, Louis-David;HU, Yun-Jin;LUO, Xuehong;TOMASZEWSKI, Miroslaw Jerzy WO2014/117274, 2014, A1 Location in patent:Paragraph 215; 219