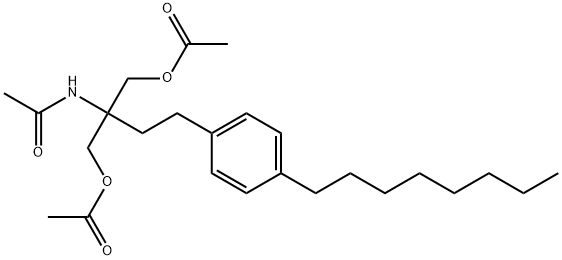
N-[1,1-Bis[(acetyloxy)methyl]-3-(4-octylphenyl)propyl]acetamide synthesis
- Product Name:N-[1,1-Bis[(acetyloxy)methyl]-3-(4-octylphenyl)propyl]acetamide
- CAS Number:162358-09-0
- Molecular formula:C25H39NO5
- Molecular Weight:433.58

108-24-7
0 suppliers
$14.00/250ML
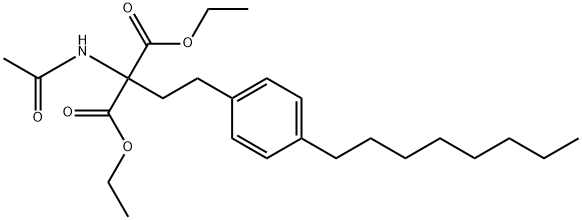
162358-08-9
129 suppliers
$225.00/50mg
![N-[1,1-Bis[(acetyloxy)methyl]-3-(4-octylphenyl)propyl]acetamide](/CAS/GIF/162358-09-0.gif)
162358-09-0
108 suppliers
$70.00/100mg
Yield: 75 g
Reaction Conditions:
Stage #1:diethyl 2-acetamido-2-[2-(4-octylphenyl)ethyl]malonate with calcium chloride in water;isopropyl alcohol at 0.2 - 0.3; for 0.00166667 h;
Stage #2: with sodium tetrahydroborate in water;isopropyl alcohol at 0 - 0.5; for 0.005 h;
Stage #3:acetic anhydride with dmap in dichloromethane at 0.15 - 0.5; for 0.002 h;Solvent;Reagent/catalyst;Temperature;
Steps:
9a Example 9a
Preparation of Compound of Formula X
Example 9a
Preparation of Compound of Formula X
In a round bottom flask 1600 ml of isopropanol and 100 gm of compound of Formula IX, obtained as in Example 8, was charged and cooled to about 20-30° C. A solution of 77 g of calcium chloride in water was added and the reaction mass was stirred for about 10 min, followed by cooling at about 0-5° C. and 61 gm sodium borohydride in about 60 min was added to above reaction mass. The reaction mass was stirred for about 240 minutes at about 0-5° C. 500 ml of 1% aq. solution of hydrogen chloride was added and stirred for about 20 min. The reaction mass was filtered and filtrate distilled out under vacuum at about 40-55° C. 1000 ml water and 750 ml ethyl acetate were added to the residue. The reaction mass was stirred for about 20 min and the organic layer was separated. The organic layer was washed with 500 ml 10% brine solution. The organic layer was distilled out under vacuum and methylene dichloride 700 ml and dimethylaminopyridine (DMAP) 10.0 g were added. The methylene dichloride layer was cooled to about 5-15° C. 71 g acetic anhydride was slowly charged and reaction mass was stirred for about 120 min. 500 ml DM water was added at about 0-30° C. and stirred for about 30 min. The organic layer was separated and washed with 500 ml of 7% aq. sodium bicarbonate solution and DM water. The organic solvent was distilled out under vacuum and 100:800 ml ethylacetate:cyclohexane were added into the residue and heated to about 60-65° C. followed by cooling to about 10° C. The solid obtained was filtered and washed with cyclohexane to get 75 g of compound of Formula X. Purity of compound: Product >99.0%. In variant of this reaction, ethanol was used as solvent instead of isopropanol.
References:
Glenmark Generics Limited;Gharpure, Milind;Narawade, Krishna;Chand, Prem;Bhirud, Shekhar US2015/18578, 2015, A1 Location in patent:Paragraph 0193
![N-[1,1-Bis[(acetyloxy)methyl]-3-[4-(1-oxooctyl)phenyl]propyl]acetamide](/CAS/20150408/GIF/249289-07-4.gif)
249289-07-4
7 suppliers
inquiry
![N-[1,1-Bis[(acetyloxy)methyl]-3-(4-octylphenyl)propyl]acetamide](/CAS/GIF/162358-09-0.gif)
162358-09-0
108 suppliers
$70.00/100mg

162358-08-9
129 suppliers
$225.00/50mg
![N-[1,1-Bis[(acetyloxy)methyl]-3-(4-octylphenyl)propyl]acetamide](/CAS/GIF/162358-09-0.gif)
162358-09-0
108 suppliers
$70.00/100mg

108-24-7
0 suppliers
$14.00/250ML
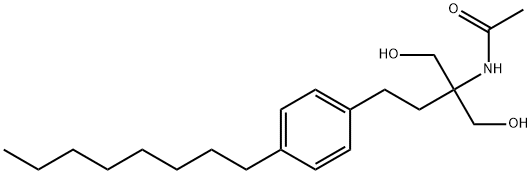
249289-10-9
99 suppliers
$105.00/50mg
![N-[1,1-Bis[(acetyloxy)methyl]-3-(4-octylphenyl)propyl]acetamide](/CAS/GIF/162358-09-0.gif)
162358-09-0
108 suppliers
$70.00/100mg

75-36-5
589 suppliers
$17.92/100G

249289-10-9
99 suppliers
$105.00/50mg
![N-[1,1-Bis[(acetyloxy)methyl]-3-(4-octylphenyl)propyl]acetamide](/CAS/GIF/162358-09-0.gif)
162358-09-0
108 suppliers
$70.00/100mg