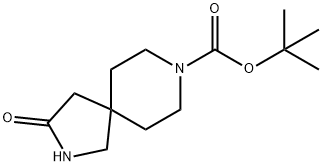
TERT-BUTYL 3-OXO-2,8-DIAZASPIRO[4.5]DECANE-8-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 3-OXO-2,8-DIAZASPIRO[4.5]DECANE-8-CARBOXYLATE
- CAS Number:169206-67-1
- Molecular formula:C13H22N2O3
- Molecular Weight:254.33
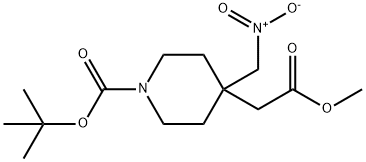
169206-66-0
![TERT-BUTYL 3-OXO-2,8-DIAZASPIRO[4.5]DECANE-8-CARBOXYLATE](/CAS/GIF/169206-67-1.gif)
169206-67-1
General procedure for the synthesis of tert-butyl 3-oxo-2,8-diazaspiro[4,5]decane-8-carboxylate from tert-butyl 4-(2-methoxy-2-oxoethyl)-4-(nitromethyl)piperidine-1-carboxylate: To a tert-butyl 4-(2-methoxy-2-oxoethyl)-4-(nitromethyl)piperidine-1-carboxylate (1.0 g, 3.2 mmol) ethanol ( 10 mL) solution was added to Raney Ni (1 g). The reaction mixture was stirred under hydrogen atmosphere at 1 atmosphere overnight. Upon completion of the reaction, the mixture was filtered through a diatomaceous earth pad to remove the catalyst and the filtrate was concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using a dichloromethane solution of 10% methanol as eluent to afford the target product tert-butyl 3-oxo-2,8-diazaspiro[4,5]decane-8-carboxylate (694 mg, 86% yield). The structure of the product was confirmed by 1H NMR (400 MHz, CD3OD): δ 3.54-3.48 (m, 2H), 3.37-3.32 (m, 2H), 3.23 (s, 2H), 2.26 (s, 2H), 1.62-1.59 (m, 4H), 1.45 (s, 9H).

169206-66-0
8 suppliers
inquiry
![TERT-BUTYL 3-OXO-2,8-DIAZASPIRO[4.5]DECANE-8-CARBOXYLATE](/CAS/GIF/169206-67-1.gif)
169206-67-1
142 suppliers
$28.00/100mg
Yield:169206-67-1 86%
Reaction Conditions:
with hydrogen;nickel in ethanol under 760.051 Torr;
Steps:
50.3
To a solution of 4-methoxycarbonylmethyl-4-nitromethyl-piperidine-1-carboxylic acid tert-butyl ester (1.0 g, 3.2 mmol) in ethanol (10 mL) was added Raney Ni (1 g). The resulting suspension was hydrogenated at 1 atm overnight. The mixture was filtered through Celite and evaporated in vacuo. The residue was purified with silica gel column chromatography (10% methanol in dichloromethane) to give the desired lactam (694 mg, 86%). 1H NMR (400 MHz, CD3OD) δ 3.54-3.48 (m, 2H), 3.37-3.32 (m, 2H), 3.23 (s, 2H), 2.26 (s, 2H), 1.62-1.59 (m, 4H), 1.45 (s, 9H).
References:
Cumbre Inc. US2006/19985, 2006, A1 Location in patent:Page/Page column 55

1462385-41-6
7 suppliers
inquiry
![TERT-BUTYL 3-OXO-2,8-DIAZASPIRO[4.5]DECANE-8-CARBOXYLATE](/CAS/GIF/169206-67-1.gif)
169206-67-1
142 suppliers
$28.00/100mg

79099-07-3
0 suppliers
$5.00/5g
![TERT-BUTYL 3-OXO-2,8-DIAZASPIRO[4.5]DECANE-8-CARBOXYLATE](/CAS/GIF/169206-67-1.gif)
169206-67-1
142 suppliers
$28.00/100mg
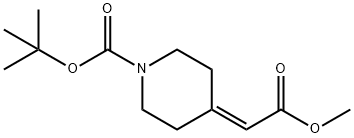
169206-65-9
56 suppliers
$496.00/5g
![TERT-BUTYL 3-OXO-2,8-DIAZASPIRO[4.5]DECANE-8-CARBOXYLATE](/CAS/GIF/169206-67-1.gif)
169206-67-1
142 suppliers
$28.00/100mg
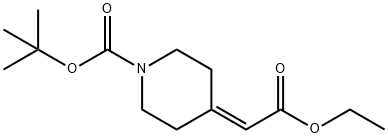
135716-08-4
142 suppliers
$5.00/250mg
![TERT-BUTYL 3-OXO-2,8-DIAZASPIRO[4.5]DECANE-8-CARBOXYLATE](/CAS/GIF/169206-67-1.gif)
169206-67-1
142 suppliers
$28.00/100mg