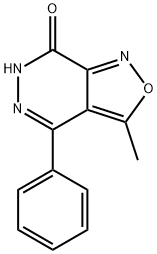
3-METHYL-4-PHENYLISOXAZOLO[3,4-D]PYRIDAZIN-7(6H)-ONE synthesis
- Product Name:3-METHYL-4-PHENYLISOXAZOLO[3,4-D]PYRIDAZIN-7(6H)-ONE
- CAS Number:17334-68-8
- Molecular formula:C12H9N3O2
- Molecular Weight:227.22
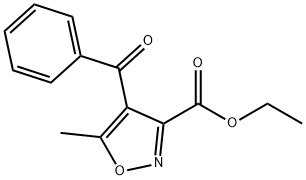
17335-06-7
6 suppliers
inquiry
![3-METHYL-4-PHENYLISOXAZOLO[3,4-D]PYRIDAZIN-7(6H)-ONE](/CAS/GIF/17334-68-8.gif)
17334-68-8
13 suppliers
inquiry
Yield:17334-68-8 84.9%
Reaction Conditions:
with hydrazine in methanol;water at 0 - 40; for 5.25 h;Product distribution / selectivity;
Steps:
1; 3
EXAMPLE 12Og (0,123 mol) of 1-phenylbutane-1 ,3-dιone and 4OmL methanol are introduced under nitrogen atmosphere, into a four-necked flask fitted with mechanical stirrer and addition funnel and the suspension is cooled down to 10-150C 24 4g of a 30% solution of sodium methoxide in methanol are added dropwise over 30mιn at 10-150C The addition funnel is washed with 2mL of methanolThe reaction mixture is stirred at 10-150C for about 30mιn and then cooled to 0-50C A solution of 20 5g (0, 135 mol) of ethyl-2-chloro-2-(hydroxyιmιno)acetate in 4OmL MeOH is added dropwise over 1 h to the reaction mixture at 0-50C, the addition funnel is washed with 2mL methanolThe reaction mixture is then warmed to 20-25°C and stirred for 2h Without any isolation or purification step, 7 4g (0,148 mol) NH2NH2 H2O are then added dropwise over 15mιn at 40°C the addition funnel is washed with 2mL of methanol and the reaction mixture is stirred at 4O0C for 4h then cooled to 20-25°CFinally 6OmL of water are added and the reaction mixture is stirred for 1 h at 20-250C then 30mιn at 0-50CThe resulting mixture is filtered, the resulting solid is washed with 2x40mL cold MeOH/H2O 1 1 and vacuum dried at 40°C overnight Yielding 22,5 g (0,099 mol) of 3- methyl-4-phenylιsoxazolo[3,4-d]pyrιdazιn-7(6H)-one (yield = 80,5%); COMPARATIVE EXAMPLE 3 The 262,86 g of impure oil from Comparative example 2 were dissolved in 550 ml of methanol, warmed to 20-250C and stirred for 2h 78,12 g (1 ,56 mol) of NH2NH2 H2O are then added dropwise over 15mιn at 4O0C, the addition funnel is washed with 2OmL of methanol and the reaction mixture is stirred at 4O0C for 4h then cooled to 20-250CFinally 48OmL of water are added and the reaction mixture is stirred for 1h at 20-250C then 30mι? at 0-50CThe resulting mixture is filtered, the resulting solid is washed with 2x40mL cold MeOH/H2O 1 1 and vacuum dried at 40°C overnight Yielding 168,2 g (0,740 mol) of 3- methyl-4-phenylιsoxazolo[3,4-d]pyrιdazιn-7(6H)-one (yield = 84,9%) HPLC-MS(+/-)= 228 MS-FIA= 228After cooling with an ice bath, a precipitate was formed and collected by filtration and washed with some pre-cooled alcoholic solventsThe combined yield of comparative examples 2 and 3 representing the yield of manufacturing 3-methyl-4-phenylιsoxazolo[3,4-d]py?dazιn-7(6H)-one in a two step reacting with isolation of the intermediate ethyl 4-benzoyl-5-methylιsoxazole-3-carboxylate is 74,0% (87,1 % X 84,9%)
References:
WO2008/107064,2008,A1 Location in patent:Page/Page column 6; 7
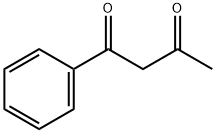
93-91-4
235 suppliers
$10.00/5g
![3-METHYL-4-PHENYLISOXAZOLO[3,4-D]PYRIDAZIN-7(6H)-ONE](/CAS/GIF/17334-68-8.gif)
17334-68-8
13 suppliers
inquiry