
(S)-4-Ethyl-4-hydroxy-8-Methoxy-6-triMethylsilanyl-1,4-dihydro-pyrano[3,4-c]pyridin-3-one synthesis
- Product Name:(S)-4-Ethyl-4-hydroxy-8-Methoxy-6-triMethylsilanyl-1,4-dihydro-pyrano[3,4-c]pyridin-3-one
- CAS Number:174092-78-5
- Molecular formula:C14H21NO4Si
- Molecular Weight:295.41
![(3R,4S)-4-Ethyl-8-Methoxy-6-triMethylsilanyl-3,4-dihydro-1H-pyrano[3,4-c]pyridine-3,4-diol](/CAS/GIF/453518-24-6.gif)
453518-24-6
0 suppliers
inquiry
![(S)-4-Ethyl-4-hydroxy-8-Methoxy-6-triMethylsilanyl-1,4-dihydro-pyrano[3,4-c]pyridin-3-one](/CAS/GIF/174092-78-5.gif)
174092-78-5
0 suppliers
inquiry
Yield:96.2 % ee
Reaction Conditions:
Stage #1: (S)-4-ethyl-3,4-dihydro-3,4-dihydroxy-8-methoxy-6-trimethylsilyl-1H-pyrano[3,4-c] pyridinewith iodine;calcium carbonate in methanol;water; for 5 h;Heating / reflux;
Stage #2: with sodium sulfite in chloroform at 20; for 0.25 h;
Steps:
30.4 Example 30; Synthesis of Tricyclic Ketone; (4); Synthesis of Compound (p)
A mixture of Compound (o) (70.2 g), iodine (183.7 g, 0.72 mol) and calcium carbonate (36.23 g, 0.36 mol) in methanol - water (10:1, 1. 0 L) was heated under ref lux for 5 hrs . After cooling, 10% sodium sulfite (1.0 L) and chloroform (1.0 L) were added to the mixture and the resulting mixture was stirred at ambient temperature for 15 min. The insoluble material was filtered by suction and the material was washed with chloroform (0.5 L). The combined organic layers of the filtrate and the washing were separated and the aqueous layer was further extracted with chloroform (0.5 L). The combined organic layers were dried over anhydrous sodium sulfate, filtered and evaporated under reduced pressure to dryness. Compound (p): umberoil, 53.6 g (overall 81% yield from Compound (m)), content: 80.4% by HPLC (see Example 22), 96.2%ee by chiral HPLC (see Example 22). 1H-NMR (400 MHz, CDCl3) δ: 0.28 (9H, s, TMS), 0.94 (3H, t, J=7.4Hz, CH2CH3), 1.76 (2H, q, J=7.4Hz, CH2CH3), 3.61 (1H, s, OH), 3.98 (3H, s, OCH,), 5.23 (1H, d, J=15.6Hz,), 5.54 (1H, d, J=15.6Hz), 7.33 (1H, s, aromatic-H).
References:
EP1378505,2004,A1 Location in patent:Page 38
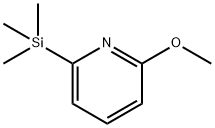
170453-55-1
4 suppliers
inquiry
![(S)-4-Ethyl-4-hydroxy-8-Methoxy-6-triMethylsilanyl-1,4-dihydro-pyrano[3,4-c]pyridin-3-one](/CAS/GIF/174092-78-5.gif)
174092-78-5
0 suppliers
inquiry
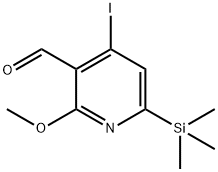
174092-75-2
0 suppliers
inquiry
![(S)-4-Ethyl-4-hydroxy-8-Methoxy-6-triMethylsilanyl-1,4-dihydro-pyrano[3,4-c]pyridin-3-one](/CAS/GIF/174092-78-5.gif)
174092-78-5
0 suppliers
inquiry
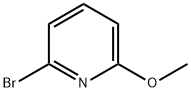
40473-07-2
277 suppliers
$7.00/1g
![(S)-4-Ethyl-4-hydroxy-8-Methoxy-6-triMethylsilanyl-1,4-dihydro-pyrano[3,4-c]pyridin-3-one](/CAS/GIF/174092-78-5.gif)
174092-78-5
0 suppliers
inquiry
![3-[((E)-But-2-enyl)oxyMethyl]-4-iodo-2-Methoxy-6-triMethylsilanyl-pyridine](/CAS/GIF/174092-76-3.gif)
174092-76-3
0 suppliers
inquiry
![(S)-4-Ethyl-4-hydroxy-8-Methoxy-6-triMethylsilanyl-1,4-dihydro-pyrano[3,4-c]pyridin-3-one](/CAS/GIF/174092-78-5.gif)
174092-78-5
0 suppliers
inquiry