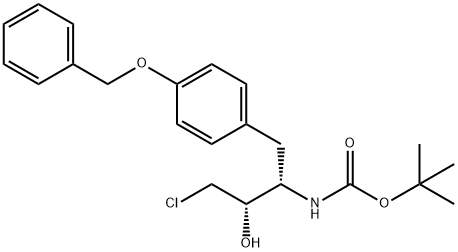
tert-butyl (2S,3S)-1-(4-(benzyloxy)phenyl)-4-chloro-3-hydroxybutan-2-ylcarbamate synthesis
- Product Name:tert-butyl (2S,3S)-1-(4-(benzyloxy)phenyl)-4-chloro-3-hydroxybutan-2-ylcarbamate
- CAS Number:174801-33-3
- Molecular formula:C22H28ClNO4
- Molecular Weight:405.92
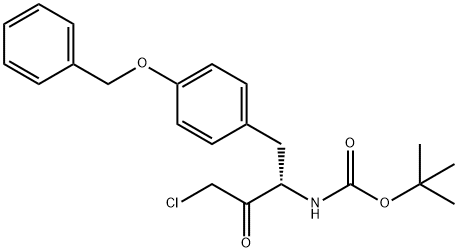
152438-62-5
20 suppliers
inquiry

174801-33-3
15 suppliers
inquiry
Yield:97.8 % de
Reaction Conditions:
with sodium tetrahydroborate in ethanol at 20; for 3 h;
Steps:
6
Ethanol (144 ml) was added and sodium borohydride (0.647 g) was added in several portions at room temperature. After stirring at room temperature for 2 hr, sodium borohydride (0.065 g) was further added and the mixture was stirred at room temperature for 1 hr. The reaction was quenched using acetic acid (1.12 ml) and the reaction mixture was heated to 70°C. This solution was allowed to cool to 20°C over 5 hr and the mixture was stirred at 20°C for 10 hr. The obtained crystals were collected by filtration and the crystals were washed with ethanol (22 ml). The crystals were further washed twice with water (45 ml, 45 ml). The obtained crystals were dried under reduced pressure to give the object (2S,3S)-N-tert-butoxycarbonyl-3-amino-4-(4-benzyloxyphenyl)-1-chloro-2-hydroxybutane (9.50 g, yield 59%). The diastereoselectivity then was (2S,3S)/(2R,3S)=98.9/1.1.1H-NMR (DMSO-d6, 400 MHz) δppm: 1.28 (s, 9H), 2.88-2.47 (m, 1H), 3.35-3.70 (m, 5H), 5.05 (s, 2H), 5.41 (d, 1H, J = 6.0 Hz), 6.65 (d, 1H, J = 8.4 Hz), 6.89 (d, 2H, J = 8.8 Hz), 7.08 (d, 2H, J = 8.8 Hz), 7.30-7.45 (m, 5H)13C-NMR(DMSO-d6, 400 MHz) δppm: 28.5, 35.0, 48.4, 54.9, 69.5, 73.1, 77.9, 114.7, 127.9, 128.1, 128.7, 130.5, 131.6, 137.7, 155.5, 157.0 mass spectrum m/e: 406 (M+H)+ [α]20D = 7.0° (c=1.0, CH2Cl2) melting point: 161-162°C
References:
EP1505065,2005,A1 Location in patent:Page 9
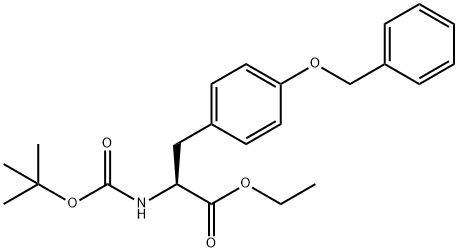
127132-32-5
26 suppliers
$17.00/250mg

174801-33-3
15 suppliers
inquiry