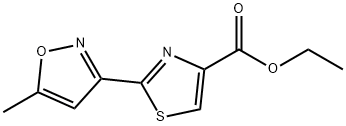
ETHYL 2-(5-METHYLISOXAZOL-3-YL)-1,3-THIAZOLE-4-CARBOXYLATE, 97 synthesis
- Product Name:ETHYL 2-(5-METHYLISOXAZOL-3-YL)-1,3-THIAZOLE-4-CARBOXYLATE, 97
- CAS Number:175277-28-8
- Molecular formula:C10H10N2O3S
- Molecular Weight:238.26
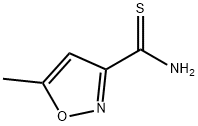
77358-26-0
42 suppliers
$70.68/250mgs:
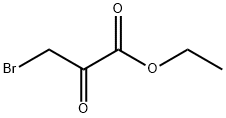
70-23-5
311 suppliers
$6.00/5g

175277-28-8
8 suppliers
inquiry
Yield:175277-28-8 53%
Reaction Conditions:
Stage #1: 5-methyl-isoxazole-3-carbothioic acid amide;ethyl Bromopyruvate in acetone at 55; for 18 h;Molecular sieve;
Stage #2: with 2,6-dimethylpyridine;trifluoroacetic acid in tetrahydrofuran at 0; for 2 h;
Steps:
272.272A
Example 272A; ethyl 2- (5-methyl-3-isoxazolyl)-1, 3-thiazole-4-carboxylate; 5-Methyl-isoxazole-3-carbothioamide (1.0 g, 7.0 mmol) was dissolved in acetone (16 mL) and treated with ethyl bromopyruvate (1 mL, 1 equivalent) and 3.9 g of powdered 3A molecular sieves. The reaction was stirred at 55°C under a nitrogen atmosphere for 18 h. The mixture was filtered and evaporated to give 1.06 g of crude material. This material was dissolved in THF (25 mL), cooled to 0°C, and treated with 2,6-lutidine (1.5 mL, 3 equivalents). Trifluoroactetic acid (0.9 mL, 1.5 equivalents) was added and stirring was continued for 2 hrs under a nitrogen atmosphere. The reaction was poured into a 1 M solution of sodium bicarbonate and extracted twice with ethyl acetate (75 mL). The combined organic layers were washed with brine, dried over magnesium sulfate, filtered and the solvent was removed by evaporation. The crude material was purified using chloroform : ethyl acetate (1: 1) to give 876 mg (53%) of the title compound.
References:
WO2005/61487,2005,A1 Location in patent:Page/Page column 157