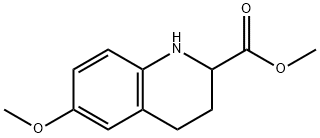
METHYL 6-METHOXY-1,2,3,4-TETRAHYDRO-QUINOLINE-2-CARBOXYLATE synthesis
- Product Name:METHYL 6-METHOXY-1,2,3,4-TETRAHYDRO-QUINOLINE-2-CARBOXYLATE
- CAS Number:176641-35-3
- Molecular formula:C12H15NO3
- Molecular Weight:221.25

176641-33-1
2 suppliers
inquiry

176641-35-3
21 suppliers
inquiry
Yield:176641-35-3 82%
Reaction Conditions:
with tris(pentafluorophenyl)borate;hydrogen in toluene at 110; under 30402 Torr; for 12 h;Autoclave;Inert atmosphere;
Steps:
Representative procedure for metal-free hydrogenation of methyl 2-quinolinecarboxylate (1a) (Table 2, entry 1):
General procedure: To a glass test tube (10 mL) was added B(C6F5) 3 (3a) (0.0102 g, 0.020 mmol), quinoline-2-carboxylic acid methyl ester (1a) (0.0749 g, 0.40 mmol) and dry toluene (1.5 mL) in a nitrogen atmosphere glovebox. The tube was then moved to a stainless-steel autoclave. After being sealed, the autoclave was purged three times with H2 and the final pressure of hydrogen was adjusted to 40 atm. The reaction mixture was stirred at 50 °C for 4 h. After cooling to ambient temperature, the solvent was removed under reduced pressure. The crude residue was purified by flash column chromatography on silica gel using petroleum ether/ethyl acetate (60/1 to 40/1) as the eluent to give methyl 1,2,3,4-tetrahydroquinoline-2-carboxylate (2a) as a light yellow oil (0.0715 g, 94% yield).
References:
Han, Caifang;Zhang;Feng, Xiangqing;Wang, Shoufeng;Du, Haifeng [Tetrahedron Letters,2018,vol. 59,# 14,p. 1400 - 1403] Location in patent:supporting information