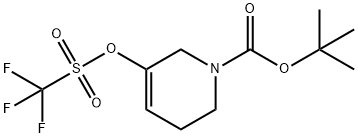
TERT-BUTYL 3-(TRIFLUOROMETHYLSULFONYLOXY)-5,6-DIHYDROPYRIDINE-1(2H)-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 3-(TRIFLUOROMETHYLSULFONYLOXY)-5,6-DIHYDROPYRIDINE-1(2H)-CARBOXYLATE
- CAS Number:180691-65-0
- Molecular formula:C11H16F3NO5S
- Molecular Weight:331.31
37595-74-7
435 suppliers
$5.00/1g
98977-36-7
410 suppliers
$6.00/1g
![tert-butyl 5-{[(trifluoromethyl)sulfonyl]oxy}-3,4-dihydropyridine-1(2H)-carboxylate(SALTDATA: FREE)](/CAS/20180713/GIF/149108-74-7.gif)
149108-74-7
12 suppliers
inquiry

180691-65-0
14 suppliers
inquiry
Yield: 75.6% , 37.8%
Reaction Conditions:
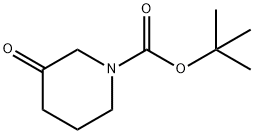
Stage #1:3-oxo-piperidine-1-carboxylic acid tert-butyl ester with lithium hexamethyldisilazane in tetrahydrofuran at -78; for 0.333333 h;
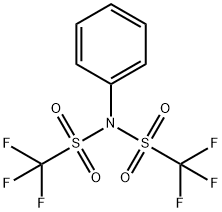
Stage #2:N,N-phenylbistrifluoromethane-sulfonimide in tetrahydrofuran at 0; for 3 h;
Stage #3: with sodium hydrogencarbonate in tetrahydrofuran;water at 0;
Steps:
P11.1.1
To a solution of tert-butyl 3-oxopiperidine-l-carboxylate (10.00 g, 50.2 mmol) in THF (50 mL) at -78 °C is added dropwise LiHMDS (55.2 mL, 55.2 mmol) (1.0 M in THF). After 20 min, a solution of l, l, l-trifluoro-N-phenyl-N-(trifluoromethylsulfonyl)methanesulfonamide (PhNTf2) (18.83 g, 52.7 mmol) in THF (50 mL) was added and the reaction warmed to 0 °C and stirred 3 hours. The reaction was quenched with saturated aqueous NaHC03 solution (1 mL) and the reaction mixture concentrated. The residue was purified through a plug of neutral AI2O3 (10% EtOAc/Hexane eluant) to give a 1: 1 mixture of tert-butyl 5- (trifluoromethylsulfonyloxy)-3,4-dihydropyridine-l(2H)-carboxylate (6.28 g, 18.96 mmol, 37.8 % yield) and tert-butyl 3-(trifluoromethylsulfonyloxy)-5,6-dihydropyridine-l(2H)-carboxylate (12.56 g, 37.92 mmol, 75.6% yield), an inseperable mixture, as a clear, light yellow oil.
References:
AMGEN INC.;ALLEN, Jennifer;HORNE, Daniel B.;HU, Essa;KALLER, Matthew R.;MONENSCHEIN, Holger;NGUYEN, Thomas T.;REICHELT, Andreas;RZASA, Robert M. WO2011/143495, 2011, A1 Location in patent:Page/Page column 84

37595-74-7
435 suppliers
$5.00/1g

98977-36-7
410 suppliers
$6.00/1g

180691-65-0
14 suppliers
inquiry

37595-74-7
435 suppliers
$5.00/1g

180691-65-0
14 suppliers
inquiry

98977-36-7
410 suppliers
$6.00/1g

180691-65-0
14 suppliers
inquiry