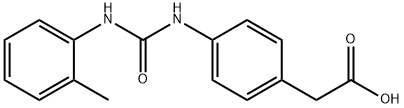
2-{4-[(2-toluidinocarbonyl)aMino]phenyl}acetic acid synthesis
- Product Name:2-{4-[(2-toluidinocarbonyl)aMino]phenyl}acetic acid
- CAS Number:181517-99-7
- Molecular formula:C16H16N2O3
- Molecular Weight:284.31
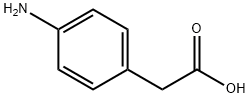
1197-55-3
424 suppliers
$13.00/25g
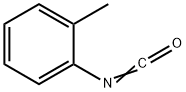
614-68-6
157 suppliers
$14.14/250mgs:
![2-{4-[(2-toluidinocarbonyl)aMino]phenyl}acetic acid](/CAS/20150408/GIF/181517-99-7.gif)
181517-99-7
20 suppliers
inquiry
Yield:181517-99-7 82%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 18 h;
Steps:
I Example I; 2-{4-[(2-toluidinocarbonyl)amino]phenyl}acetic acid
[0334] To a solution of 2-(4-aminophenyl)acetic acid (108.8 g, 0.72 mol) in CH2Cl2 (1.0 l) and triethylamine (120 ml) was added a solution of 2-methylphenyl isocyanate (90.5 ml, 0.72 mol) in CH2Cl2 (500 ml) dropwise at r.t. After stirring for 18 h at r.t., water (2.5 l) and CH2Cl2 (2.0 l) were added and the layers were separated. The organic layer was extracted with water (3×400 ml). The combined aqueous layers were concentrated to 3.0 l and acidified to pH 2 by the addition of concentrated aqueous HCl. The precipitate was collected by filtration, washed with cold water and dried in an exsiccator over concentrated H2SO4 affording 166.5 g (82%) white solid. M.p. 205-206° C.; TLC (CH2Cl2/MeOH 9:1): Rf 0.14. 1H-NMR (400 MHz, D6-DMSO): 12.21 (br s, 1H), 9.11 (s, 1H), 8.00 (s, 1), 7.83 (d, 7.6 Hz, 11, 7.40 (d, 8.5 Hz, 2H), 7.17-7.12 (m, 4), 6.96-6.92 (m, 111, 3.48 (s, 2H), 2.24 (s, 3H).
References:
US2003/232868,2003,A1 Location in patent:Page 14