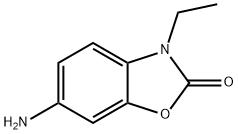
6-amino-3-ethyl-1,3-benzoxazol-2(3H)-one(SALTDATA: HCl) synthesis
- Product Name:6-amino-3-ethyl-1,3-benzoxazol-2(3H)-one(SALTDATA: HCl)
- CAS Number:184159-08-8
- Molecular formula:C9H10N2O2
- Molecular Weight:178.19
32418-07-8
11 suppliers
$75.00/1g

184159-08-8
6 suppliers
inquiry
Yield:184159-08-8 747 mg
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in tetrahydrofuran;ethanol;
Steps:
29A 6-amino-3-ethyl-1,3-benzoxazol-2(3H)-one
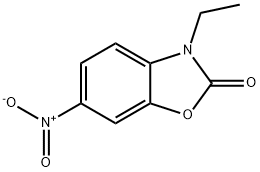
1.00 g (4.80 mmol) of 3-ethyl-6-nitro-1,3-benzoxazol-2(3H)-one [for preparation see: WO 2007/120339 A1, 37-38] was initially charged in 32.5 ml of ethanol, then 51 mg (0.05 mmol) of palladium (10% on activated carbon) were added and the mixture was hydrogenated at standard hydrogen pressure overnight. Subsequently, the reaction mixture was filtered through kieselguhr and the filtrate was concentrated. The residue was taken up in 50.0 ml of an ethanol/ THF mixture (1:1), 50 mg (0.05 mmol) of palladium (10% on activated carbon) were added and the mixture was hydrogenated further at standard hydrogen pressure overnight. The reaction mixture was filtered again through kieselguhr, the filtercake was washed with ethanol and the filtrate was concentrated. The residue was subjected to extractive stirring in ethanol, and the solid was filtered off and washed with ethanol. After drying under high vacuum, this gave 747 mg of the target compound (83% of theory). LC-MS (Method 3): Rt=0.29 min; MS (ESIpos): m/z=179 (M+H)+. 1H NMR (400 MHz, DMSO-d6): δ [ppm]=1.21 (t, 3H), 3.74 (q, 2H), 4.99-5.05 (m, 2H), 6.42 (dd, 1H), 6.55 (d, 1H), 6.94 (d, 1H).
References:
US2015/148340,2015,A1 Location in patent:Paragraph 0632; 0633; 0634; 0635