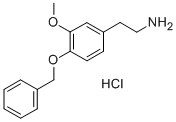
3-Methoxy-4-(benzyloxy)phenethylamine Hydrochloride synthesis
- Product Name:3-Methoxy-4-(benzyloxy)phenethylamine Hydrochloride
- CAS Number:1860-57-7
- Molecular formula:C16H20ClNO2
- Molecular Weight:0
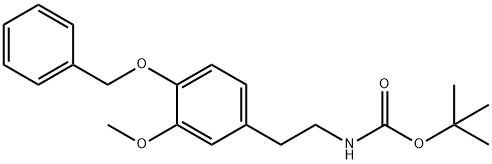
23428-81-1
1 suppliers
inquiry

1860-57-7
20 suppliers
inquiry
Yield:1860-57-7 64%
Reaction Conditions:
Stage #1: 3-Methoxy-4-benzyloxy-N-tert.-butyloxycarbonyl-phenaethylaminwith trifluoroacetic acid in dichloromethane at 0; for 0.666667 h;
Stage #2: with sodium hydrogencarbonate in dichloromethane;water;Cooling with ice;
Stage #3: with hydrogenchloride in diethyl ether;
Steps:
2.1
Trifluoroacetic acid (20 ml) was gently added to a dichloromethane solvent (20 ml) having the white solid (16.8 mmol, 6.0 g) at 0° C. After stirring the mixture for 40 minutes, the mixture solution was gently placed in a sodium bicarbonate solution with ice. The mixture was extracted with a diethylether solvent, which was then removed, and dissolved in chloroform to be neutralized with saturated sodium bicarbonate solution, and the solvent was removed. The resultant material was treated with 1M HCl in diethylether (20 ml) to give a white hydrochloride salt, intermediate 6 (1.32 g, 64%) [1H NMR (CDCl3, 200 MHz) δ8.24 (br s, 2H), 7.28-7.45 (m, 5H), 6.96 (d, J=8.0 Hz, 1H), 6.90 (d, J=1.8 Hz, 1H), 6.73 (dd, J=8.0, 1.8 Hz, 1H), 5.04 (s, 1H), 3.77 (s, 3H), 2.96-3.03 (m, 2H), 2.80-2.88 (m, 2H); 13C NMR (CDCl3, 50 MHz) δ149.3, 146.6, 137.2, 130.3, 128.2, 127.6, 127.5, 120.5, 114.1, 113.0, 70.1, 55.6, 32.4; MS (CI): 258 (M+), 241 (100), 228, 91].
References:
US2010/217003,2010,A1 Location in patent:Page/Page column 6
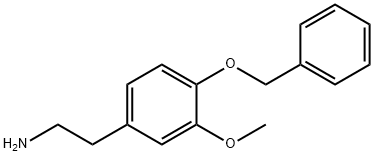
22231-61-4
26 suppliers
inquiry

1860-57-7
20 suppliers
inquiry
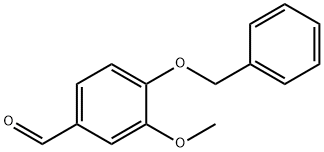
2426-87-1
254 suppliers
$8.00/5g

1860-57-7
20 suppliers
inquiry
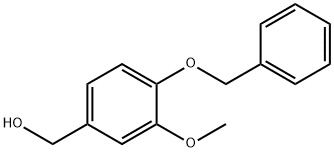
33693-48-0
73 suppliers
$18.00/1g

1860-57-7
20 suppliers
inquiry
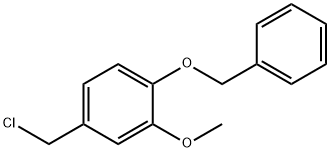
33688-50-5
12 suppliers
inquiry

1860-57-7
20 suppliers
inquiry