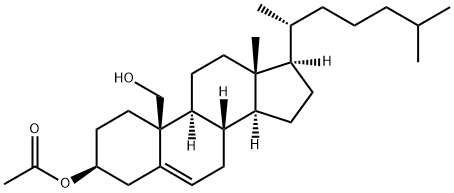
19-HYDROXYCHOLESTEROL 3-ACETATE synthesis
- Product Name:19-HYDROXYCHOLESTEROL 3-ACETATE
- CAS Number:750-59-4
- Molecular formula:C29H48O3
- Molecular Weight:444.69
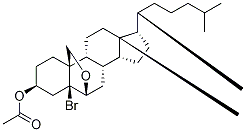
1258-07-7
10 suppliers
$109.90/5mg

750-59-4
16 suppliers
$452.41/5MG
Yield:750-59-4 89%
Reaction Conditions:
with water;acetic acid;zinc at 20; for 75 h;
Steps:
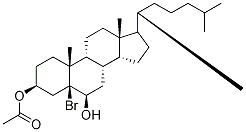
To a stirred solution of bromo ether S3 (1.42 g, 2.71 mmol) in acetic acid and water (15:1, 19.2mL) at rt was added activated zinc dust (3.54 g, 54.2 mmol). After stirring for 21 h, acetic acid (9.00 mL), water (0.60 mL), and activated zinc dust (1.77 g, 27.1 mmol) were supplemented. After 54 h, CH2Cl2 (35 mL) was added to the mixture, and insoluble materials were filtered off. The filtrate wasextracted with CH2Cl2 (30 mL). The extract was washed with brine (50 mL), dried over Na2SO4, and concentrated in vacuo. The residue (1.19 g) was purified by silica gel column chromatography (25 g,EtOAc/hexane = 2:8) to give homoallylic alcohol S4 (1.08 g, 89%) as a white solid: Rf 0.38 (Et2O/hexane = 1:1); 1H NMR (400 MHz, CDCl3) δ 5.74 (d, J = 5.2 Hz, 1H), 4.61 (m, 1H), 3.80 (d, J= 11.2 Hz, 1H), 3.59 (d, J = 11.2 Hz, 1H), 2.39 (m, 1H), 2.23 (m, 1H), 2.03-1.90 (m, 6H), 1.85-1.74 (m, 3H), 1.61-1.45 (m, 6H), 1.33-1.21 (m, 4H), 1.19-0.99 (m, 9H), 0.92-0.87 (m, 5H), 0.83 (dd, J =6.6, 2.0 Hz, 6H), 0.70 (s, 3H). Other data were identical to those reported.2
References:
Oikawa, Yuya;Uchiyama, Daiki;Shirasawa, Takuya;Oikawa, Masato;Ishikawa, Yuichi [Tetrahedron Letters,2016,vol. 57,# 35,p. 3949 - 3951] Location in patent:supporting information
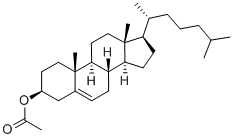
604-35-3
274 suppliers
$56.00/25g

750-59-4
16 suppliers
$452.41/5MG
1258-35-1
12 suppliers
$110.00/25mg

750-59-4
16 suppliers
$452.41/5MG
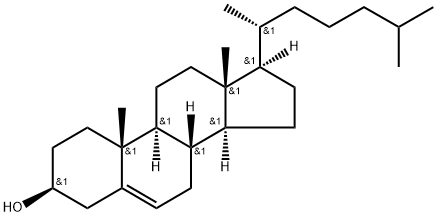
57-88-5
871 suppliers
$10.00/1g

750-59-4
16 suppliers
$452.41/5MG
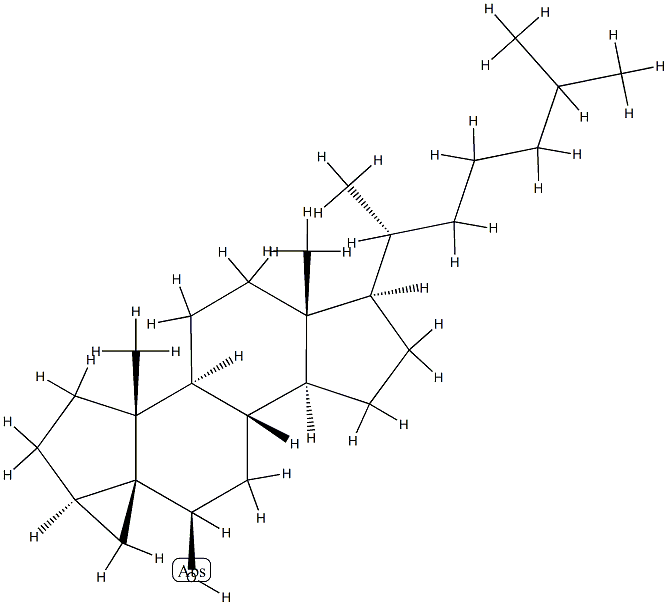
465-54-3
1 suppliers
inquiry

750-59-4
16 suppliers
$452.41/5MG