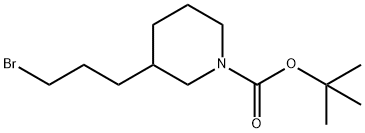
tert-Butyl 3-(3-bromopropyl)-piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 3-(3-bromopropyl)-piperidine-1-carboxylate
- CAS Number:193629-30-0
- Molecular formula:C13H24BrNO2
- Molecular Weight:306.24
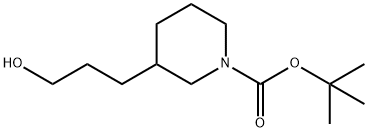
163210-22-8
25 suppliers
$82.00/50 mg

193629-30-0
18 suppliers
$83.00/50 mg
Yield: 11.6 grams (70%)
Reaction Conditions:
with pyridine;bromine;triphenylphosphine in dichloromethane;water
Steps:
15 Preparation of (3'S) 3-[1-(t-butoxycarbonyl)piperidin-3-yl]propyl bromide STR20
PREPARATION 15 Preparation of (3'S) 3-[1-(t-butoxycarbonyl)piperidin-3-yl]propyl bromide STR20 To a cold (0° C.) solution of triphenylphosphine (19.95 g, 76 mmol) in anhydrous methylene chloride (110 ml) was added bromine dropwise until the solution turned pale yellow. A few crystals of triphenylphosphine were added to the mixture to bring the color back to white. To this mixture was added a suspension of (3'S) 3-[1-(t-butoxycarbonyl)piperidin-3-yl]propanol (13.2 g, 54.4 mmol) and pyridine (8.0 g, 76 mmol) in dry methylene chloride (110 ml). The resulting mixture was stirred for five hours while warming to room temperature. The reaction was stopped by adding water (200 ml). The organic fraction was extracted with methylene chloride (3*200 ml). The combined organic layer was washed with water (2*200 ml), then brine (1*100 ml), and then dried over sodium sulfate. The solvents were removed in vacuo to provide a light brownish crude product, which was further purified by flash chromatography to yield 11.6 grams (70%) of the desired title product. NMR and IR were consistent with the title product.
References:
Eli Lilly and Company US5776931, 1998, A