
TERT-BUTYL 4-(5-CYANOPYRIDIN-2-YLOXY)PIPERIDINE-1-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 4-(5-CYANOPYRIDIN-2-YLOXY)PIPERIDINE-1-CARBOXYLATE
- CAS Number:194668-38-7
- Molecular formula:C16H21N3O3
- Molecular Weight:303.36
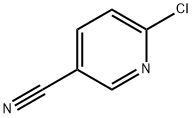
33252-28-7
439 suppliers
$6.00/1g
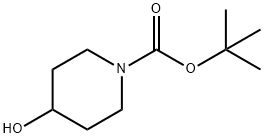
109384-19-2
420 suppliers
$5.00/1g

194668-38-7
10 suppliers
inquiry
Yield:194668-38-7 94%
Reaction Conditions:
Stage #1: 1-(tert-Butyloxycarbonyl)-4-piperidinolwith sodium hydride in DMF (N,N-dimethyl-formamide) at 20 - 50; for 1.41667 h;
Stage #2: 6-chloronicotinonitrile in DMF (N,N-dimethyl-formamide) at 60;
Steps:
6.1
Example 6 6-(1-Pyridin-2-ylmethyl-piperidin-4-yloxy)-nicotinamide Step 1 4-(5-Cyano-pyridin-2-yloxy)-piperidine-1-carboxylic acid tert-butyl ester Add dropwise a solution of 4-hydroxy-piperidine-1-carboxylic acid tert-butyl ester (1210 mg, 6.01 mmol) in DMF (1.8 mL) to a suspension of NaH (360 mg, 9.02 mmol) in DMF (7.2 mL). Stir at room temperature for 45 min then at 50°C for additional 40 min. Add a solution of 6-chloro-nicotinonitrile (1000 mg, 7.22 mmol) in DMF (3.6 mL) dropwise and stir overnight at 60°C. Cool down the reaction mixture and evaporate the solvent. Wash the residue with water (10) and extract with EtOAc/hex (2/1, 15 mL). Combine the organic layers and dry over sodium sulfate. Filter and concentrate. Purify the resulting residue through an SCX column. Further purified by chromatography [CH2C12/NH3 (2. 0M in methanol) 20/1] to provide the title compound (1.73 g, 94%).
References:
WO2005/61442,2005,A1 Location in patent:Page/Page column 40-41

918535-59-8
1 suppliers
inquiry

194668-38-7
10 suppliers
inquiry