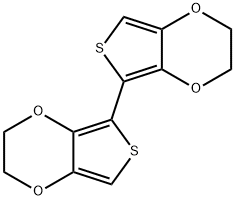
2,3-DIHYDRO-5-(2,3-DIHYDROTHIENO[3,4-B][1,4]DIOXIN-5-YL)THIENO[3,4-B][1,4]DIOXINE synthesis
- Product Name:2,3-DIHYDRO-5-(2,3-DIHYDROTHIENO[3,4-B][1,4]DIOXIN-5-YL)THIENO[3,4-B][1,4]DIOXINE
- CAS Number:195602-17-6
- Molecular formula:C12H10O4S2
- Molecular Weight:282.34
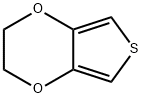
126213-50-1
![2,3-DIHYDRO-5-(2,3-DIHYDROTHIENO[3,4-B][1,4]DIOXIN-5-YL)THIENO[3,4-B][1,4]DIOXINE](/CAS/GIF/195602-17-6.gif)
195602-17-6
The general procedure for the synthesis of 2,2',3,3'-tetrahydro-5,5'-dithieno[3,4-b][1,4]dioxohexene using 3,4-ethylenedioxythiophene (EDOT) as a starting material was as follows: to an anhydrous tetrahydrofuran (THF, 10 cm3) solution of EDOT (0.995 g, 7.0 mmol) was slowly added, at -78 °C, n Butyl lithium (n-BuLi, 1.34 M hexane solution, 5.37 cm3, 1.01 equiv). After 2 hours of reaction, anhydrous copper chloride (CuCl?, 0.941 g, 7.0 mmol) was added all at once. Subsequently, the reaction mixture was slowly warmed to room temperature and stirred continuously for 12 hours. Upon completion of the reaction, distilled water (10 cm3) was added to the mixture to terminate the reaction. The product was extracted with dichloromethane (CH?Cl?, 2 x 50 cm3), and the combined organic phases were dried over anhydrous magnesium sulfate (MgSO?) and concentrated under reduced pressure to remove the solvent. The crude product was purified by column chromatography (solvent mixture of hexane and dichloromethane with eluent ratio of 1:1) to give a white solid target product. The yield was 0.951 g with 48% yield. Elemental analysis results (C12H10O4S2): calculated value C 51.06%, H 3.57%; measured value C 51.01%, H 3.49%. Mass spectrum (ES+) m/z: calculated value (C12H11O4S2)+ was 283.00988; measured value was 283.00978. nuclear magnetic resonance (NMR) hydrogen spectrum (1H NMR, 400MHz): δ 6.27 (s, 2H, thiophene group H), 4.22-4.34 (m, 8H). NMR carbon spectrum (13C{1H} NMR, 100.6 MHz): δ 141.63,137.43,100.00,99.94,65.41,65.01. Infrared spectra (IR) major absorption peaks: 2944,1465,1170,1056 cm?1.

126213-50-1
455 suppliers
$8.00/5g
![2,3-DIHYDRO-5-(2,3-DIHYDROTHIENO[3,4-B][1,4]DIOXIN-5-YL)THIENO[3,4-B][1,4]DIOXINE](/CAS/GIF/195602-17-6.gif)
195602-17-6
52 suppliers
$51.00/100mg
Yield: 99%
Reaction Conditions:
Stage #1:3,4-(ethylenedioxy)thiophene with n-butyllithium;N,N,N,N,-tetramethylethylenediamine in tetrahydrofuran at -10; for 0.5 h;Metallation;
Stage #2: with iron(III)-acetylacetonate in tetrahydrofuran for 6 h;Heating;Oxidation;
References:
Zhu, S. Sherry;Swager, Timothy M. [Journal of the American Chemical Society,1997,vol. 119,# 51,p. 12568 - 12577]
![Thieno[3,4-b]-1,4-dioxin, 2,3-dihydro-5-iodo-](/CAS/20200515/GIF/219621-78-0.gif)
219621-78-0
1 suppliers
inquiry
![2,3-DIHYDRO-5-(2,3-DIHYDROTHIENO[3,4-B][1,4]DIOXIN-5-YL)THIENO[3,4-B][1,4]DIOXINE](/CAS/GIF/195602-17-6.gif)
195602-17-6
52 suppliers
$51.00/100mg
![tributyl(2,2',3,3'-tetrahydro-[5,5'-bithieno[3,4-b][1,4]dioxin]-7-yl)stannane](/CAS/20210111/GIF/862681-10-5.gif)
862681-10-5
2 suppliers
inquiry
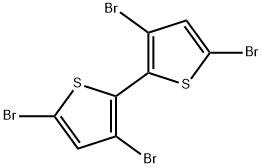
125143-53-5
138 suppliers
$5.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![2,3-DIHYDRO-5-(2,3-DIHYDROTHIENO[3,4-B][1,4]DIOXIN-5-YL)THIENO[3,4-B][1,4]DIOXINE](/CAS/GIF/195602-17-6.gif)
195602-17-6
52 suppliers
$51.00/100mg
![tributyl(2,2',3,3'-tetrahydro-[5,5'-bithieno[3,4-b][1,4]dioxin]-7-yl)stannane](/CAS/20210111/GIF/862681-10-5.gif)
862681-10-5
2 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![2,3-DIHYDRO-5-(2,3-DIHYDROTHIENO[3,4-B][1,4]DIOXIN-5-YL)THIENO[3,4-B][1,4]DIOXINE](/CAS/GIF/195602-17-6.gif)
195602-17-6
52 suppliers
$51.00/100mg