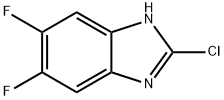
1H-Benzimidazole,2-chloro-5,6-difluoro-(9CI) synthesis
- Product Name:1H-Benzimidazole,2-chloro-5,6-difluoro-(9CI)
- CAS Number:142356-63-6
- Molecular formula:C7H3ClF2N2
- Molecular Weight:188.56
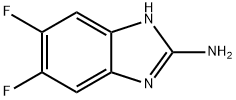
142356-62-5
28 suppliers
inquiry

142356-63-6
17 suppliers
inquiry
Yield:142356-63-6 73%
Reaction Conditions:
Stage #1: with tert.-butylnitrite;copper dichloride in acetone at 20; for 0.333333 h;
Stage #2: 5,6-difluoro-1H-benzo[d]imidazol-2-amine in acetone; for 2 h;Heating / reflux;
Stage #3: with hydrogenchloride in water;acetone at 20;
Steps:
2 Step 2.
The procedure used was similar to that reported in J. Med. Chem. 40:811-818, 1997. To a mixture of copper(II) chloride (795 mg, 5.91 mmol) in acetone (20 mL) was added tert-butyl nitrite (0.53 mL, 4.43 mmol). The reaction mixture was stirred at rt for 20 minutes, 5,6-difluoro-1H-benzimidazol-2-amine (500 mg, 2.96 mmol) was then added, and the mixture was heated at reflux for 2 h (with additional portions of tert-butyl nitrite added every 0.5 h). The reaction mixture was then cooled to rt, treated with 2 N HCl, and extracted with ethyl acetate. The organic phase was dried over sodium sulfate and concentrated in vacuo to afford 2-chloro-5,6-difluoro-1H-benzimidazole (580 mg, 73%), which was used without further purification in the next step. LC-MS m/z 189.2 (MH+), ret. time 1.96 min; 1H NMR (300 MHz, DMSO-d6) δ 7.62 (t, 2H), 13.5 (br s, 1H).
References:
US2004/224997,2004,A1 Location in patent:Page 34
176244-21-6
26 suppliers
inquiry

142356-63-6
17 suppliers
inquiry
76179-40-3
204 suppliers
$5.00/250mg

142356-63-6
17 suppliers
inquiry
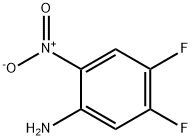
78056-39-0
193 suppliers
$5.00/1g

142356-63-6
17 suppliers
inquiry