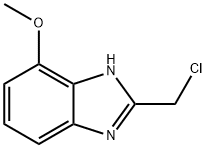
1H-Benzimidazole,2-(chloromethyl)-4-methoxy-(9CI) synthesis
- Product Name:1H-Benzimidazole,2-(chloromethyl)-4-methoxy-(9CI)
- CAS Number:405173-83-3
- Molecular formula:C9H9ClN2O
- Molecular Weight:196.63
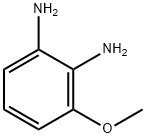
37466-89-0
94 suppliers
$30.00/100mg

405173-83-3
5 suppliers
inquiry
Yield:405173-83-3 386 mg (68%)
Reaction Conditions:
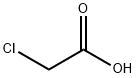
with chloroacetic acid in hydrogenchloride;
Steps:
99 Compound 99:
A solution of 2,3-diaminoanisole (400 mg, 2.89 mmol) and chloroacetic acid (557 mg, 6 mmol) in 4 N HCl was heated to 105° C. for 16 hours. The mixture was then cooled, neutralized (to a pH of 8) with aqueous sodium bicarbonate, and extracted twice with dichloromethane. The organic fractions were then dried over anhydrous sodium sulfate, filtered and concentrated. The residue was purified by silica gel flash chromatography using a 1:1 mixture of hexanes:ethyl acetate as an eluent to give 1H-2-chloromethyl-4-methoxybenzimidazole as a yellow foam in a yield of 386 mg (68%). 1H NMR (CDCl3) δ 3.96 (s, 3H), 4.85 (s, 2H), 6.71 (m, 1H), 7.19 (m, 2H).
References:
US2004/19058,2004,A1
555-03-3
9 suppliers
$25.00/1g

405173-83-3
5 suppliers
inquiry
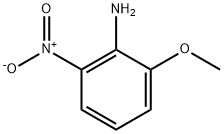
16554-45-3
143 suppliers
$16.00/1g

405173-83-3
5 suppliers
inquiry