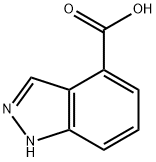
1H-INDAZOLE-4-CARBOXYLIC ACID synthesis
- Product Name:1H-INDAZOLE-4-CARBOXYLIC ACID
- CAS Number:677306-38-6
- Molecular formula:C8H6N2O2
- Molecular Weight:162.15
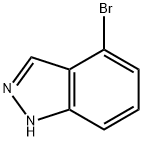
186407-74-9

677306-38-6
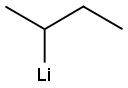
The general procedure for the synthesis of indazole-4-carboxylic acid from 4-bromo-1H-indazole was as follows: sodium hydride (60% dispersed in mineral oil, 1.11 eq.) was added batchwise to an anhydrous tetrahydrofuran (7 L/mol) solution of 4-bromo-1H-indazole (1.00 eq.) at room temperature. The resulting solution was stirred at room temperature for 30 minutes and subsequently cooled to -60°C. A cyclohexane solution of 1.3 M sec-butyl lithium (2.1 eq.) was slowly added while keeping the internal temperature below -50 °C. Stirring of the reaction mixture was continued at -50 °C for 2 hours. Subsequently, a steady stream of anhydrous carbon dioxide gas was passed into the reaction mixture for 1 hour. While maintaining the carbon dioxide gas stream, the reaction mixture was allowed to gradually warm up to room temperature. Brine (6 L/mol) was added and the pH of the mixture was adjusted with concentrated hydrochloric acid to 5. The mixture was extracted with warm ethyl acetate (3 x 8 L/mol), the organic phases were combined, washed with a small amount of brine, dried over anhydrous sodium sulfate and concentrated. Finally, indazole-4-carboxylic acid was purified by silica gel column chromatography or crystallization.

186407-74-9
320 suppliers
$5.00/1g

677306-38-6
165 suppliers
$8.00/250mg
Yield: 55%
Reaction Conditions:
Stage #1:4-bromoindazole with sodium hydride in tetrahydrofuran;mineral oil at 20; for 0.5 h;
Stage #2: with sec.-butyllithium in cyclohexane at -50; for 2 h;
Stage #3: with carbon dioxide for 1 h;
Steps:
71
To a solution of bromoindazole (1.00 eqiv) in anhydrous tetrahydrofuran (7 L/mol) at room temperature was added sodium hydride (60% in mineral oil, 1.11 eqiv) in several portions. The resulting solution was maintained for 30 min at room temperature and was then cooled to-60 C.A 1.3 M solution of sec-butyllithium in cyclohexane (2.1 eqiv) was added to the reaction mixture while maintaining the internal temperature below-50 C. The mixture was maintained for an additional 2 h at -50 C. A steady stream of anhydrous carbon dioxide was bubbled through the reaction mixture for 1 h. The flow was continued while the reaction mixture was allowed to warm to room temperature. Brine (6 L/mol) was added and the pH of the mixture was adjusted to 5 with concentrated hydrochloric acid. The mixture was extracted with warm ethyl acetate (3 x 8 L/mol) and the combined extracts were washed with small volume of brine, dried over anhydrous sodium sulfate, and concentrated. The product was purified by chromatography on silica gel or by crystallization
References:
Memory Pharmaceuticals Corporation WO2004/29050, 2004, A1 Location in patent:Page 103;104
192945-49-6
135 suppliers
$12.00/1g

677306-38-6
165 suppliers
$8.00/250mg
598-30-1
190 suppliers
$41.89/50ml

677306-38-6
165 suppliers
$8.00/250mg
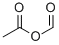
2258-42-6
47 suppliers
inquiry

186407-74-9
320 suppliers
$5.00/1g

677306-38-6
165 suppliers
$8.00/250mg
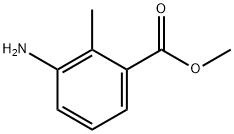
18583-89-6
221 suppliers
$6.00/1g

677306-38-6
165 suppliers
$8.00/250mg