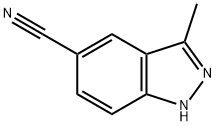
1H-Indazole-5-carbonitrile, 3-methyl- synthesis
- Product Name:1H-Indazole-5-carbonitrile, 3-methyl-
- CAS Number:267875-55-8
- Molecular formula:C9H7N3
- Molecular Weight:157.17
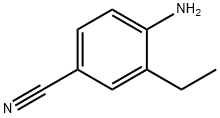
170230-87-2

267875-55-8
F. Synthesis of 3-methyl-1H-indazole-5-carbonitrile. An aqueous solution (2 mL) of sodium nitrite (3.6 g, 53 mmol) was slowly added dropwise to a solution of glacial acetic acid (150 mL) of 4-amino-3-ethylbenzonitrile (7.0 g, 48 mmol). The reaction mixture was stirred at room temperature for 16 hours and then concentrated under reduced pressure to one-third of the original volume, followed by the addition of water (200 mL). The pH of the aqueous phase was adjusted with saturated sodium bicarbonate solution to 10. The aqueous phase was extracted with ethyl acetate (3 × 200 mL), the organic extracts were combined and dried with anhydrous magnesium sulfate. After concentration of the organic phase under reduced pressure, the crude product was purified by rapid chromatography on silica gel (eluent: 0-45% hexane solution of ethyl acetate) to afford 3-methyl-1H-indazole-5-carbonitrile as an orange solid (3.3 g, 21 mmol, 30% yield). Mass spectrum (ESI) m/z 158.2 [M + H]+.
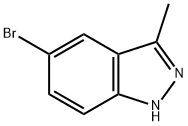
552331-16-5
190 suppliers
$25.00/1g
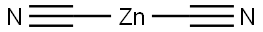
557-21-1
106 suppliers
$12.00/5g

267875-55-8
44 suppliers
inquiry
Yield:267875-55-8 700 mg
Reaction Conditions:
with tetrakis-(triphenylphosphine)-palladium in N,N-dimethyl-d6-formamide at 100; for 1 h;Inert atmosphere;
Steps:
Step-1. 3-Methyl-1H-indazole-5-carbonitrile
Pd(PPh3)4 (1.09 g, 0.95 mmol) and Zn(CN)2 (662.0 mg, 5.64 mmol) were added to a solution of 5-bromo-3-methyl- 1 /7-indazolc (1.00 g, 4.74 mmol) in DMF (15.00 mL). The resulting solution was stirred at 100 °C for Ih under nitrogen. The solids were then filtered out, and the solution was diluted with water, extracted with dichloromethane, and the organic layers were concentrated under vacuum. The residue was purified by silica gel chromatography (EtOAc/petroleum ether, 1/1 v/v) to obtain the title compound (700.0 mg) as an oil. LCMS (ESI): 158.1 [M+H]+.
References:
WO2022/99144,2022,A1 Location in patent:Paragraph 287-288

170230-87-2
136 suppliers
$10.00/250mg

267875-55-8
44 suppliers
inquiry
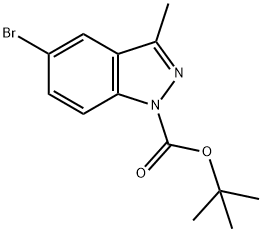
552331-49-4
40 suppliers
$93.00/100mg
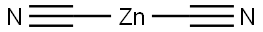
557-21-1
106 suppliers
$12.00/5g

267875-55-8
44 suppliers
inquiry
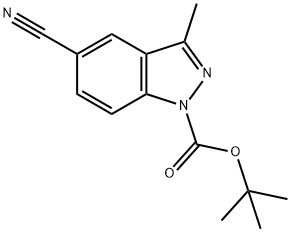
1015068-77-5
0 suppliers
inquiry